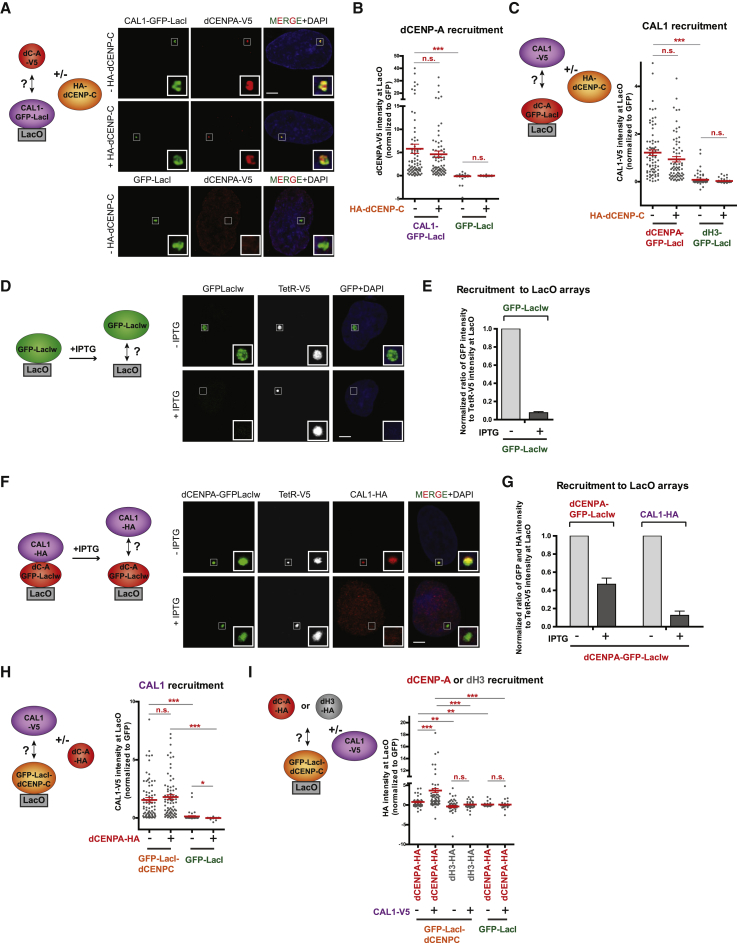
Figure 3.
dCENP-C Recruits the CAL1/dCENP-A Complex to Chromatin
(A) Experimental scheme and representative IF images of dCENP-A-V5 recruitment to the LacO arrays by CAL1-GFP-LacI or GFP-LacI, +/− HA-dCENP-C, in U2OS cells.
(B) Quantitation of normalized dCENP-A-V5 mean intensity at LacO upon tethering of CAL1-GFP-LacI or the control GFP-LacI, +/− HA-dCENP-C (for % cells see Figure S2A).
(C) Experimental scheme and quantitation of normalized CAL1-V5 mean intensity at LacO upon tethering of GFP-LacI-tagged dCENP-A or dH3, +/− HA-dCENP-C (for % cells see Figure S2C).
(D) Experimental scheme and representative IF images of GFP-LacIw recruitment to the LacO arrays, +/− IPTG treatment in U2OS cells.
(E) Quantitation of normalized GFP-LacIw mean intensity at LacO, +/− IPTG treatment.
(F) Experimental scheme and representative IF images of CAL1-HA recruitment to the LacO arrays by dCENP-A-GFP-LacIw, +/− IPTG treatment in U2OS cells.
(G) Quantitation of normalized dCENP-A-GFP-LacIw and CAL1-HA mean intensities at LacO, +/− IPTG treatment.
(H) Experimental scheme and quantitation of normalized CAL1-V5 mean intensity at LacO upon tethering of GFP-Lac-dCENP-C or GFP-LacI, +/− dCENP-A-HA (for % cells see Figure S2H).
(I) Experimental scheme and quantitation of normalized HA-tagged dCENP-A or dH3 mean intensities at LacO upon tethering of GFP-Lac-dCENP-C or GFP-LacI, +/− CAL1-V5 (for % cells see Figure S2J; see also Figure S2).
Scale bar, 5 μm. Insets show magnification of the boxed regions. Error bars show SEM (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; n.s., not significant).