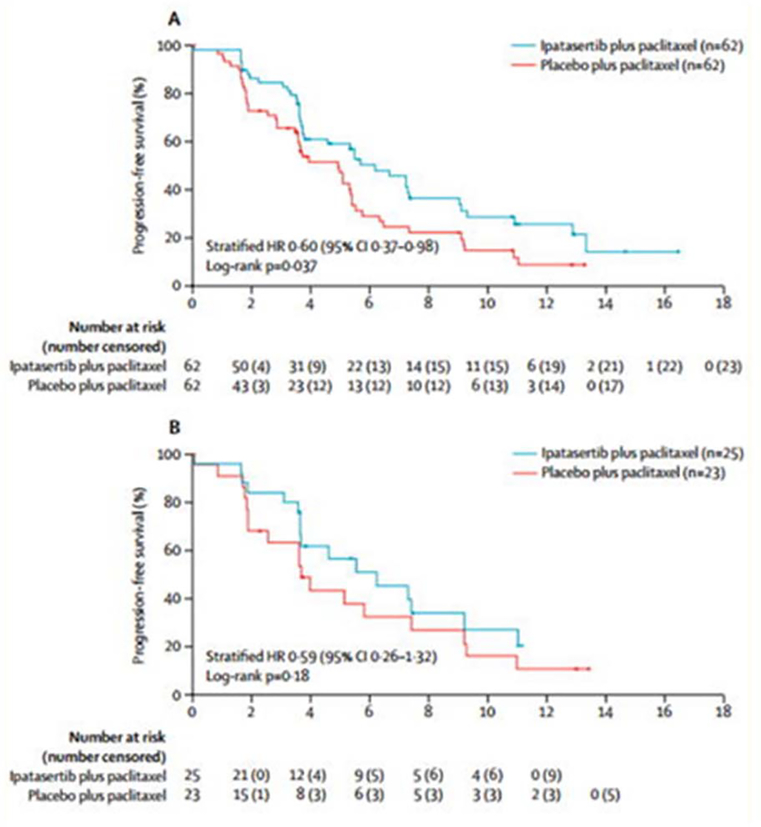
Figure 3.
Progression-free survival (PFS) in randomized clinical trial of ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple negative breast cancer. A) PFS in the intention-to-treat population unselected by biomarker, and B) PFS in patients with tumors bearing PIK3CA/AKT1/ phosphatase and tensin homolog (PTEN) alterations. Figure was reprinted with permission from “Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial” by Sung-Bae Kim, 2017. The Lancet Oncology, Volume 18, Pages 1360–1372.