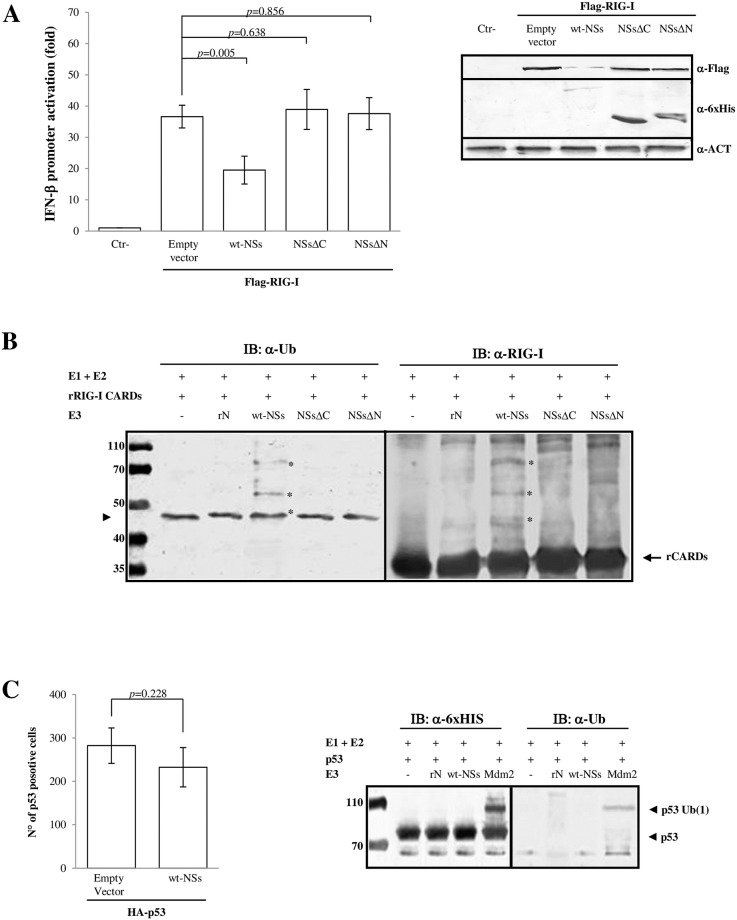
Fig 1. NSs acts as E3 ubiquitin ligase on RIG-I CARDs.
Toscana virus NSs inhibits the IFN-β promoter activation through the RIG-I signalling pathway mediating its degradation. Lenti-X 293T cells were transfected (A) with IFN-β promoter-driven FireFly Luciferase (p125-FFLuc) reporter plasmid, expression plasmid encoding FLAG-RIG-I and wild-type (wt-) NSs, as well as deleted NSs expression plasmids, as indicated. In addition, pSV40-RenLuc plasmid was added as internal control. Luciferase activity was analyzed at 48h post-transfection by the Dual-Luciferase Reporter assay as described by the manufacturer (Promega). Relative luciferase activities were measured as fold induction (relative to the basal level of reporter genes in the presence of empty vector after normalization with co-transfected RenLuc activities). Values represent means of triplicate independent experiments ± standard deviations (SD). Representative western blot showing the protein expression levels in the reporter gene assay samples was done on whole cell extracts, resolved by sodium dodecyl sulfate(SDS)-polyacrylamide gel electrophoresis (PAGE) and analyzed by immunoblotting with the FLAG-RIG-I, 6xHis-NSs and β-Actin specific antibodies and densitometric analysis (Supplement data 2). (B) Recombinant proteins were used in the in vitro ubiquitination assay using UbcH5b/c as E2 and wt-rNSs, rNSsΔC or rNSsΔN as source of E3 ubiquitin ligase. The negative controls were represented by rRIG-I CARDs tested with the ubiquitination reagents except for E3 Ub ligase or TOSV nucleoprotein (rN) in place of E3 Ub ligase. The ubiquitinated rRIG-I CARDs were detected with anti-ubiquitin (left panel) or anti-RIG-I (right panel) antibodies. The ubiquitinated forms of rCARDS (indicated by asterisk) are present only in the samples containing wt-rNSs, as demonstrated by mass-spectrometry (S4 Fig). The band indicated by arrowhead (left panel) corresponding to the ubiquitinated-E2 (Ub-E2) intermediate present in all the tested samples, comigrates with the monoubiquitinated-rRIG-I CARDs in presence of rNSs. (C) NSs does not affect p53 expression in cells transfected with HA-p53 and 6xHis-NSs expressing plasmids (left panel). Immunofluorescence was performed with indicated specific antibodies; positive cells were counted. The bars depict the average number of p53 positive cells in the presence or absence of wt-NSs. The mean ± SD of three independent experiments is shown. The specificity of the E3 ubiquitin ligase activity of wt-NSs was evaluated in the in vitro ubiquitination assay using UbcH5b/c as E2 and recombinant p53 protein as acceptor target for ubiquitination (right panel). Poly-ubiquitinated forms were detected by anti-6xHis tag and anti-ubiquitin antibodies. Higher molecular weight bands corresponding to ubiquitinated p53 were detected only in the reaction supplemented with Mdm2 E3 ubiquitin ligase, but not in the samples containing wt-rNSs or rN.