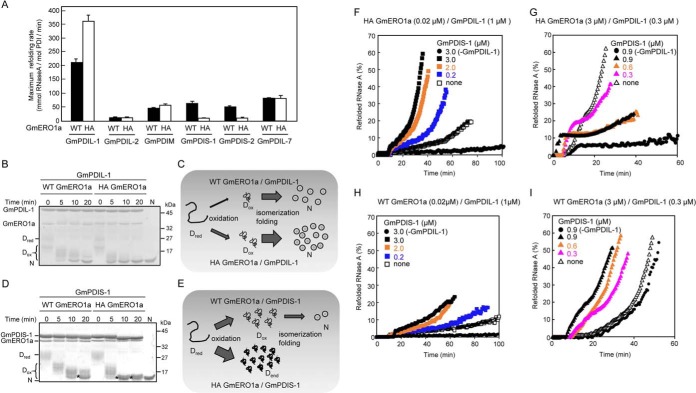
Figure 7.
Effect of the loss of regulation of GmERO1a activity on oxidative folding catalyzed by PDI family proteins. A, reduced and denatured RNase A (8 μm) was incubated with 3 μm of each PDI family protein in the presence of 1 μm WT GmERO1a (black bars) or C121A/C146A-hyperactive (HA) mutant GmERO1a (white bars) at 25 °C, after which the recovered RNase A activity was assayed. Data are represented as the mean ± S.E. of n = 3 experiments. B and D, formation of disulfide bonds in reduced and denatured RNase A during refolding catalyzed by GmPDIL-1 (B) or GmPDIS-1 (D) described in A. The reaction was quenched with 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid. Proteins in the reaction mixture were analyzed by nonreducing SDS-PAGE. Dred, reduced and denatured RNase A; Dox, denatured RNase A with nonnative disulfides; asterisks, denatured RNase A with dead-end disulfide bonds; N, native RNase A. C, model of RNase A refolding catalyzed by GmPDIL-1 in the presence of WT or HA GmERO1a. GmPDIL-1 transfers nonnative disulfide bonds to Dred in the presence of WT or HA GmERO1a. More Dox is generated in the presence of HA GmERO1a than in the presence of WT GmERO1a. Because Dox is rapidly folded into the native form, accompanied by the isomerization of disulfide bonds catalyzed by GmPDIL-1, more N is formed in the presence of HA GmERO1a than in the presence of WT GmERO1a. E, model of RNase A refolding catalyzed by GmPDIS-1 in the presence of WT or HA GmERO1a. Denatured RNase A with dead-end disulfide bonds (Dend) is generated by GmPDIS-1 in the presence of HA GmERO1a than in the presence of WT GmERO1a. F–I, cooperative refolding of RNase A catalyzed by GmPDIL-1 and GmPDIS-1 in the presence of a nonregulated (F and G) or regulated (H and I) supply of oxidizing equivalents. Reduced and denatured RNase A (8 μm) was incubated without (−GmPDIL-1) or with 1 μm (F and H) or 0.3 μm GmPDIL-1 (G and I) and GmPDIS-1 at the indicated concentrations in the presence of 0.02 μm HA (F), 3 μm HA (G), or 0.02 μm WT (H), or 3 μm WT (I) GmERO1a.