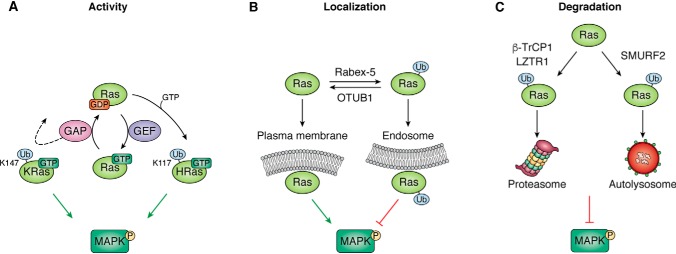
Figure 3.
Ubiquitination controls multiple interactions of RAS. A, RAS activity is regulated by ubiquitination. Primary sites of monoubiquitination occur at residues 147 in KRAS, whereas in HRAS it is at 117. Monoubiquitination of KRAS at 147 up-regulates RAS activity through a GAP defect leading to enhanced MAPK activation. In contrast, monoubiquitination of HRAS at 117 induces fast exchange and activates RAS in a GEF-independent manner. B, RAS localization is regulated by ubiquitination. Rabex-5 promotes mono- and diubiquitination of HRAS and NRAS resulting in endosome localization and reduced MAPK signaling. The deubiquitinase, OTUB1, removes ubiquitin from RAS and promotes plasma membrane localization and MAPK signaling. C, ubiquitination by LZTR1, β-TrCP1, and SMURF2 promotes RAS degradation through proteasome and autolysosomes resulting in reduced MAPK signaling.