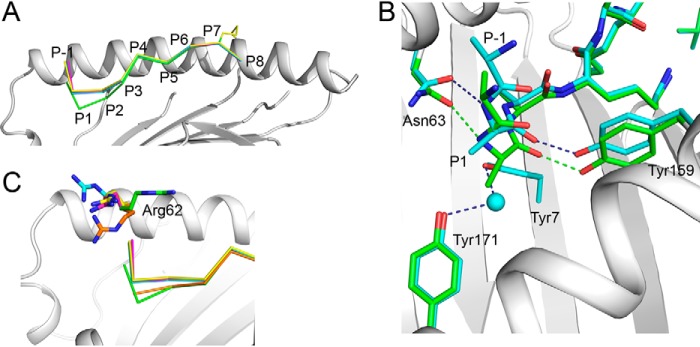
Figure 2.
Binding of a nested set of N-terminally extended peptides (R(N-Me)A)(RA)n-1AAKKKYCL into HLA-B*0801E76C. A, superimposition of the structures of 10-mer (green), 12-mer (cyan), 14-mer (magenta), and 20-mer (yellow) (R(N-Me)A)(RA)n-1AAKKKYCL, shown against the α1-helix of HLA-B*0801E76C/10-mer complex (light gray). The backbone conformations of the peptides are very similar except between P1 and P3. B, superimposition of the structures of the 10-mer (green) and 12-mer (cyan) (R(N-Me)A)(RA)n-1AAKKKYCL, shown within the A pocket of HLA-B*0801E76C/10-mer complex (light gray). A water molecule (cyan) occupies the A pocket in the 12-mer structure. Hydrogen bonds are shown as green and dark blue dashed lines for the 10- and 12-mer, respectively. C, same as in A, showing that in the canonical structure of HLA-B*0801-bound GGKKKYKL (orange), the configuration of Arg62 “closes” the A pocket. Arg62 changes its position significantly upon binding the 10-mer (green) (see also Fig. 1D), 12-mer (cyan), 14-mer (magenta), and 20-mer (yellow).