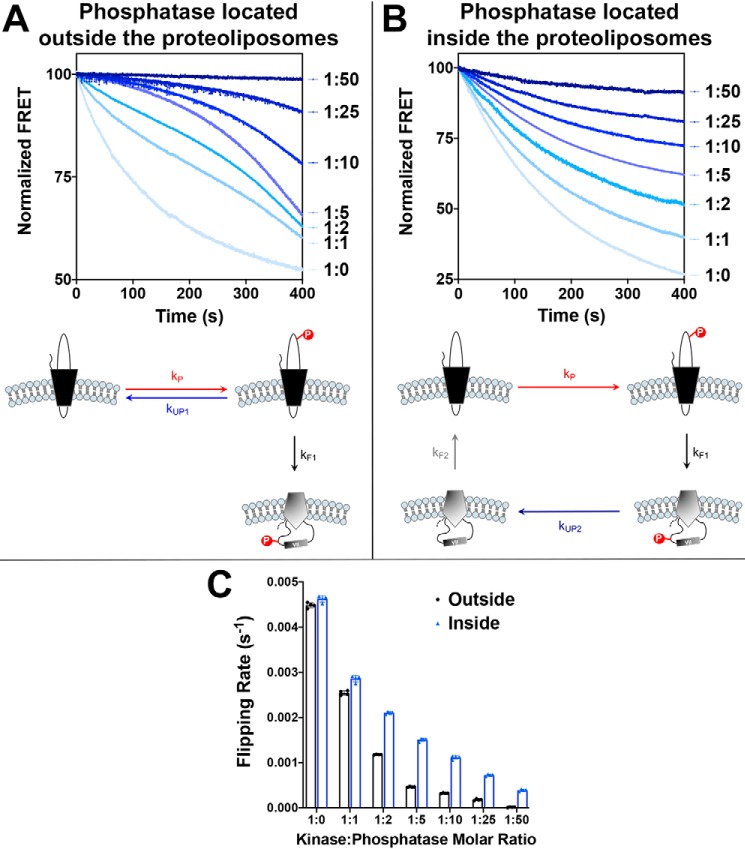
Figure 3.
Determination of the effects of dephosphorylation rates on the dephosphorylation-induced topological switch of a membrane protein. LacY template −2/0/−2 with a single Trp replacement in EMD C6, V331C labeled with IAEDANS and containing a single engineered PDK1 sequence in EMD C6 was reconstituted in proteoliposomes made of E. coli native lipids. A and B, real-time FRET measurements monitoring phosphorylation-induced topological switch of LacY in the presence of various concentrations of phosphatase located either outside of proteoliposomes (A) or encapsulated in proteoliposomes (B). Molar kinase:phosphatase ratios are indicated next to the measured kinetics. Data represent the averaged normalized fluorescence expressed as the ratio FMeas/F0. Schematics of the measured events are shown, with the varied rates of dephosphorylation highlighted. C, the flipping rates, kF1, determined from linear regression fits of the first 30 s of the data shown in A and B. In all cases, the data represent mean values ± S.D. from four experimental replicates.