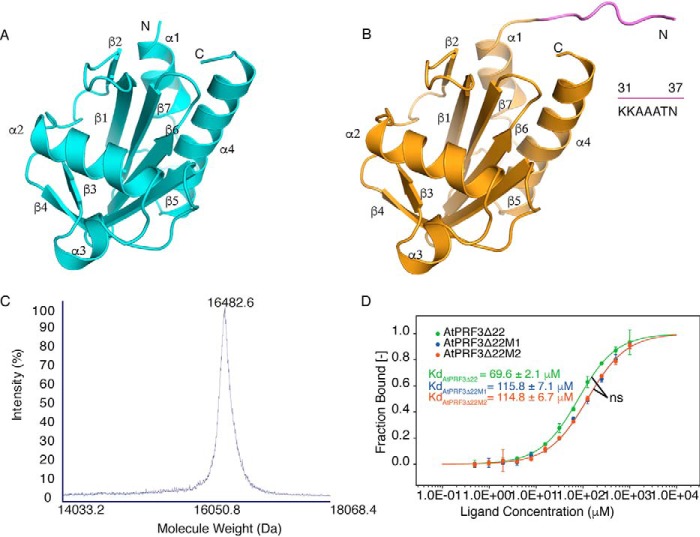
Figure 4.
Structures and characterization of AtPRF3Δ37 and AtPRF3Δ22. A, crystal structure of AtPRF3Δ37 colored in cyan. B, crystal structure of AtPRF3Δ22 in orange with the extra amino acids (KKAAATN) colored in violet. C, the MALDI-TOF-MS result of AtPRF3Δ22M2 sample for characterizing protein size and integrity. D, thermophoresis binding curves of AtPRF3Δ22, AtPRF3Δ22M1, and AtPRF3Δ22M2 titrated against AtFH1 Poly-P from experiments of three biological replicates. The dissociated constants are shown, respectively. ns indicates no significance between all three sets of affinity data of AtPRF3Δ22, AtPRF3Δ22M1, and AtPRF3Δ22M2.