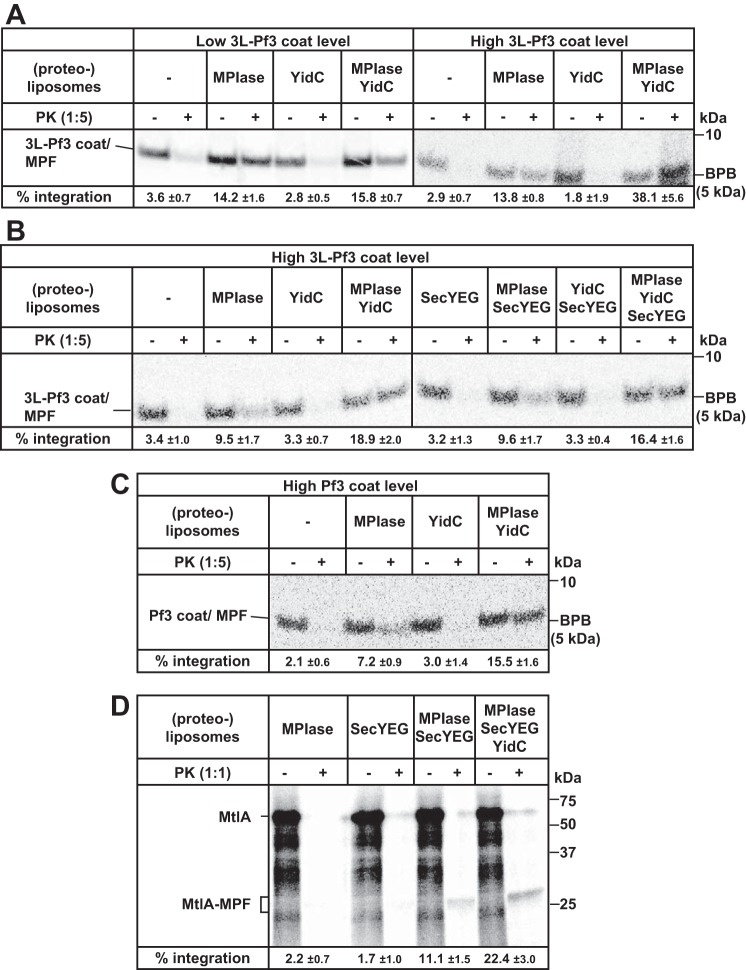
Figure 3.
YidC accelerates MPIase-dependent integration into reconstituted proteoliposomes. A, 3L-Pf3 coat integration is accelerated by YidC when the substrate level is high. (Proteo)liposomes containing MPIase and/or YidC were subjected to the integration assay using 3L-Pf3 coat as a substrate, as described in Fig. 1B. Cold methionine (0.3 mm) was added in the right half (high 3L-Pf3 level), whereas it was not added in the left half (low 3L-Pf3 coat level). The integration activities are indicated at the bottom. B, SecYEG is not involved in 3L-Pf3 coat integration. 3L-Pf3 coat was in vitro synthesized in the presence of (proteo)liposomes containing MPIase, YidC, and/or SecYEG, as specified. To increase the level of 3L-Pf3 coat, 0.3 mm methionine was added (high 3L-Pf3 coat level). The integration activities are indicated at the bottom. C, Pf3 coat integration is accelerated by YidC. Pf3 coat was in vitro synthesized in the presence of (proteo)liposomes containing MPIase and/or YidC, as specified, in the presence of 0.3 mm methionine (high 3L-Pf3 coat level). The integration activities are indicated at the bottom. D, MtlA integration depends on both MPIase and SecYEG, and is accelerated by YidC. MtlA was in vitro synthesized in the presence of (proteo)liposomes containing MPIase, SecYEG, and/or YidC, as specified. Cold methionine (30 μm) was also added. The integration activities are indicated at the bottom. All the integration activities with S.D. were calculated for three independent experiments.