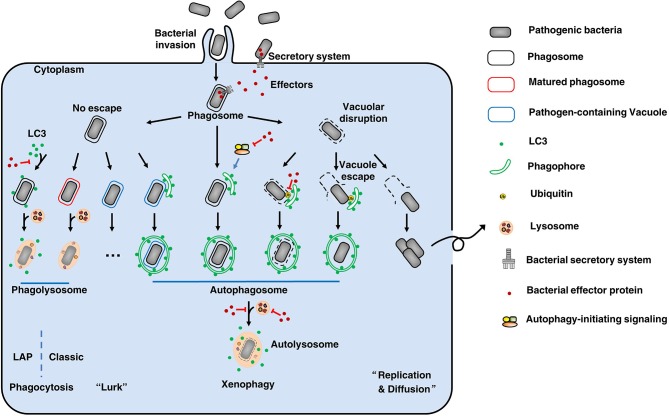
Figure 1.
The fate of intracellular bacteria. After entry into host cells, bacteria are localized to internalization vacuoles, which are designated as phagosomes To survive, bacteria employ diverse means to escape or subvert host cellular defenses, especially using its secretion systems and effectors. By various ways, some bacteria (like Salmonella Typhimurium) can damage the phagosomes and then escape to the cytoplasm, where can obtain nutrients to replicate and to diffuse. On the other hand, to clean up the bacteria remaining in phagosomes, the phagosomes will be mature and fuse with lysosomes to form phagolysosomes where the bacteria are degraded. This's the classic phagocytosis. To prevent phagocytosis-mediated bacterial killing, bacteria (such as Mycobacterium tuberculosis) can modify the phagosomes to form pathogen-containing vacuoles, thus avoiding fusion with lysosomes. These bacteria will lurk to wait for opportunities for their survival. Therefore, xenophagy plays a key role in cell resistance to these crafty bacteria by clearing pathogen-containing vacuoles, escaped pathogens, damaged vacuoles, and pathogen-containing phagosomes. During this process, above targets are enclosed by phagophores. Then, the phagophores elongate to form autophagosomes that fuse with lysosomes to form autolysosomes where the bacteria are eliminated. Notably, LC3-associated phagocytosis (LAP) can recruit the autophagy marker protein LC3 to pathogen-containing phagosomes, and the subsequent fusion of these phagosomes with lysosomes results in pathogen digestion. Additionally, there are other unmentioned cross-talk between xenophagy and phagocytosis. Back to our theme, effectors-autophagy interactions. Using effectors delivered by secretion systems, bacteria are able to interfere with autophagy-initiating signaling, modify LC3 protein, avoid autophagosome-lysosome fusion, affect lysosome function, and deubiquitinate ubiquitinated substrate around intracellular bacteria, etc. Thus, bacteria can suppress or subvert autophagic responses for their survival. Overall, there is a constant battle between bacterial evasion mechanisms and host cellular defenses, and the fate of intracellular bacteria is determined by the outcome of this battle.