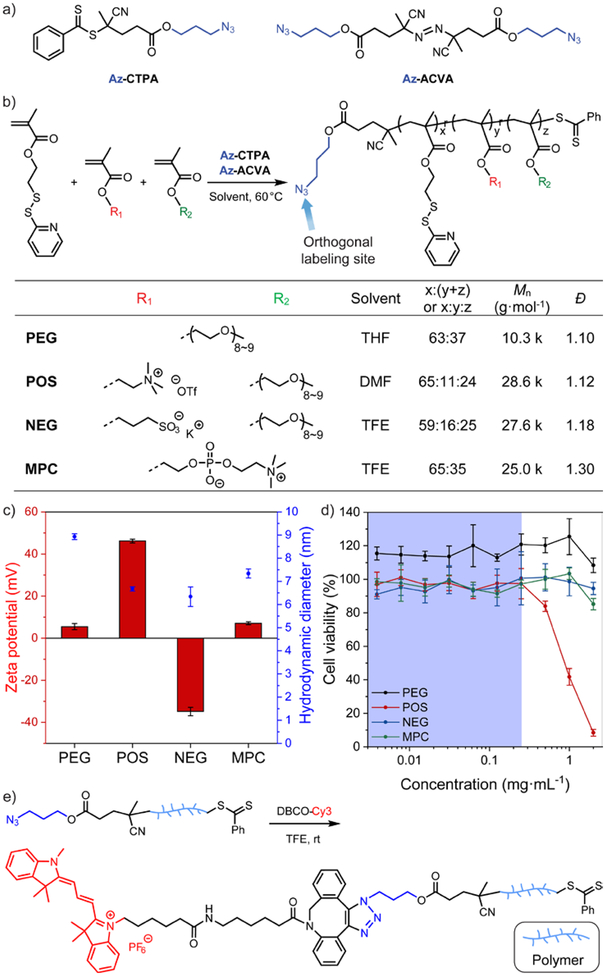
Figure 1.
(a) Chemical structure of the azido-derivative of chain transfer agent (Az-CTPA) and radical initiator (Az-ACVA) for RAFT polymerization. (b) Synthetic scheme and characterization of the amphiphilic polymer library. (c) Zeta potential and hydrodynamic diameter of the amphiphilic polymers. N = 3. The amphiphilic polymers were formulated with 10 mol% (vs. PDS units) of dithiothreitol to form the polymeric assemblies. (d) HeLa cell viability evaluation with amphiphilic polymers at different dosage. N = 4. In each figure, error bars represent the standard deviation of replicates. (e) Synthetic scheme of the orthogonal end-group labeling strategy for amphiphilic polymers.