Figure 6.
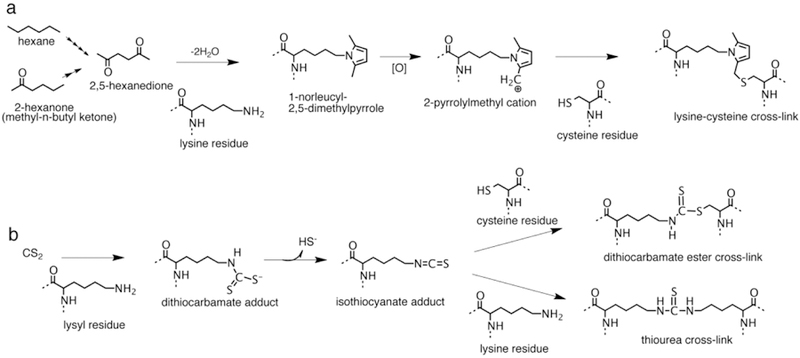
Protein cross-linking pathways for hexane, methyl-n-butyl ketone and carbon disulfide (CS2). (a) Hexane and methyl-n-butyl ketone are metabolized to 2,5-hexanedione through multiple steps of −1 oxidation. 2,5-Hexanedione reacts with protein lysyl residues to generate a 2,5-dimethylpyrrole adduct. In the presence of oxygen the pyrrole adduct oxidizes to a cationic species susceptible to nucleophilic addition by protein amino and sulfhydryl groups producing protein cross-links. Reaction of the oxidized pyrrole adduct with a cysteine residue to produce a possible lysine-cysteine cross-link is shown. (b) Carbon disulfide reacts with protein lysyl residues to form a monoalkyldithiocarbamate adduct. The dithiocarbamate adduct undergoes facile elimination of sulfhydryl ion creating an electrophilic isothiocyanate adduct. The isothiocyanate adduct then undergoes nucleophilic addition by protein sulfhydryl or amino groups to generate dithiocarbamate ester or thiourea protein cross-links.
