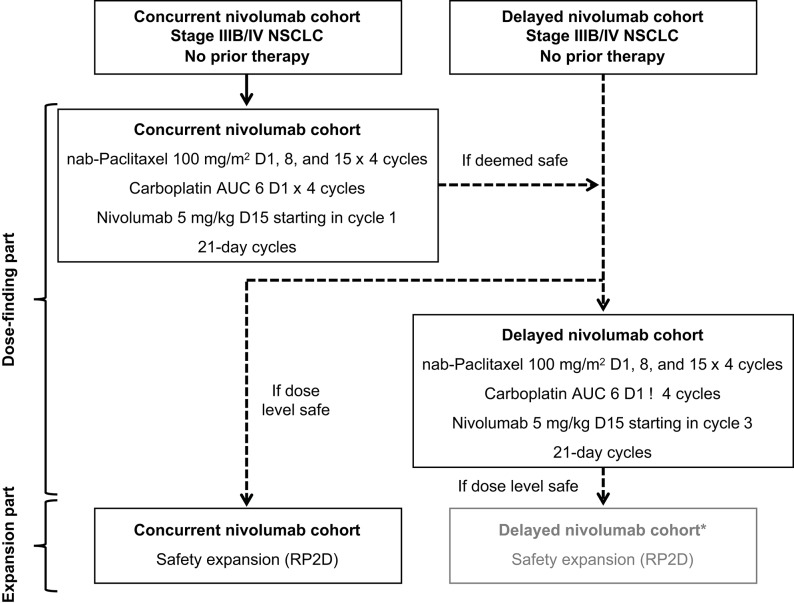
Figure 1.
Study design. *The expansion part of the delayed cohort was not pursued because the survival outcomes in the dose-finding part of this cohort were not as encouraging as those in the concurrent cohort and the safety profile with concurrent nivolumab administration was acceptable. AUC, area under the curve; NSCLC, non–small-cell lung cancer; RP2D, recommended part 2 dose.