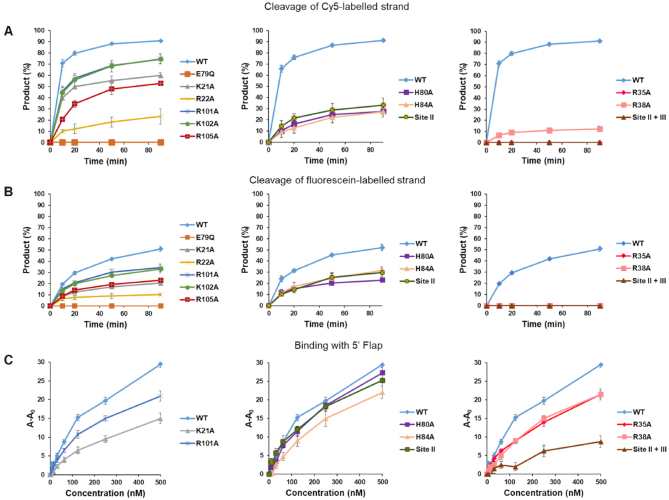
Figure 5.
Activity and DNA binding by point mutations of Cg-Slx1 in complex with Cg-Slx4CCD. (A) Time course reactions of 5′-flap cleavage by Cg-Slx1–Slx4CCD variants. Cleavage of the Cy5-labeled flap DNA strand was monitored. Cleavage products were resolved on denaturing gels and visualized by a fluorescence scanner. The amount of product was measured by gel densitometry and is reported as a percentage of total densitometry counts of all of the bands. (Left) Activity of site III mutants (Cg-Slx1K21A-Slx4CCD, Cg-Slx1R22A-Slx4CCD, Cg-Slx1R101A-Slx4CCD, Cg-Slx1K102A-Slx4CCD, Cg-Slx1R105A-Slx4CCD). (Middle) Activity of site II mutants (Cg-Slx1H80A-Slx4CCD, Cg-Slx1H84A-Slx4CCD, Cg-Slx1H80A/H84A-Slx4CCD). Mutant Cg-Slx1H80A/H84A-Slx4CCD is referred to as the Site II mutant. (Right) Activity of site I mutants (Cg-Slx1R35A-Slx4CCD, Cg-Slx1R38A-Slx4CCD) and the Cg-Slx1H80A/H84A/K21A/R22A/R101A/K102A-Slx4CCD variant with mutations in site II and site III (Site II + III). Wild type Cg-Slx1WT-Slx4CCD and catalytically inactive Cg-Slx1E79Q-Slx4CCDwere used as positive and negative controls, respectively. (B) As in (A) but cleavage of the fluorescein-labeled continuous strand was monitored. The representative gels that are related to the activity tests are shown in Supplementary Figure S5. All of the experiments were repeated three times. All variants were tested in each repetition and the results are split into separate panels for clarity. The plot for wild-type protein is repeated in each panel for easier comparisons. Error bars represent the standard deviation over three experiments. (C) Binding of 5′-flap DNA by selected site I, site II, and site III Cg-Slx1–Slx4 mutants. Binding was studied using fluorescence anisotropy. Cy5-labeled DNA strand was monitored. Descriptions of the various mutants are provided in Supplementary Table S3. Y-axis is labeled A-A0, where A is the measured anisotropy and A0 is the anisotropy of the DNA alone.