Figure 8.
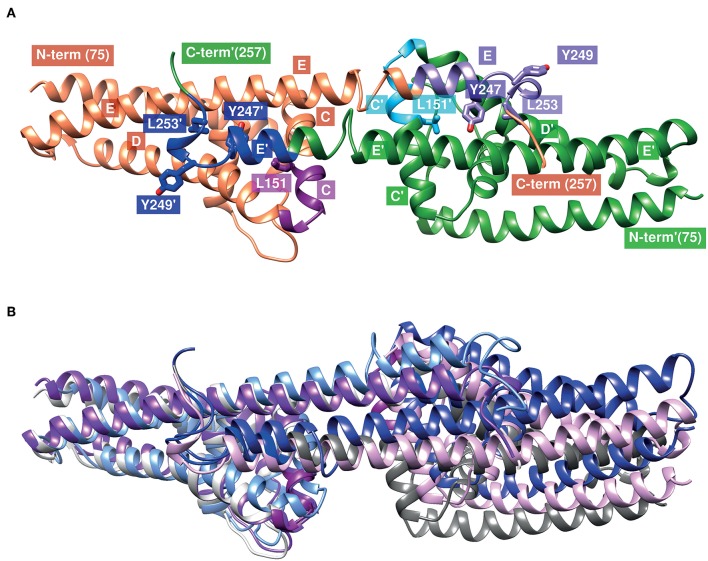
Structural model of the CspA of B. mayonii MN14-1420 dimer and superimposed structures of CspA of B. burgdorferi. (A) The two subunits are represented as orange or green ribbons, regions involved in binding of FHL-1 or FH are colored magenta (151–159) and violet (240–246) in the orange subunit and light blue and blue in the green subunit, respectively. Helices are marked by a capital letter, mutated residues are shown in sticks, and an apostrophe denotes the second subunit. Molecular graphics images are produced using the UCSF Chimera (92). (B) Superimposed structures of the CspA homodimer of both orthologs (1w33, 4bl4, 5a2u) show the different orientations of the C-terminal helix and the second subunit. The subunits in the structures of 1w33, 4bl4, and 5a2u are colored light-and dark-gray, light- and dark-magenta and light- and dark-blue, respectively. We modeled the CspA of B. mayonii MN14-1420 structure according to the crystal structure of CspA of B. burgdorferi B31 (1w33) using the Swiss-Model automated comparative protein modeling server (93).