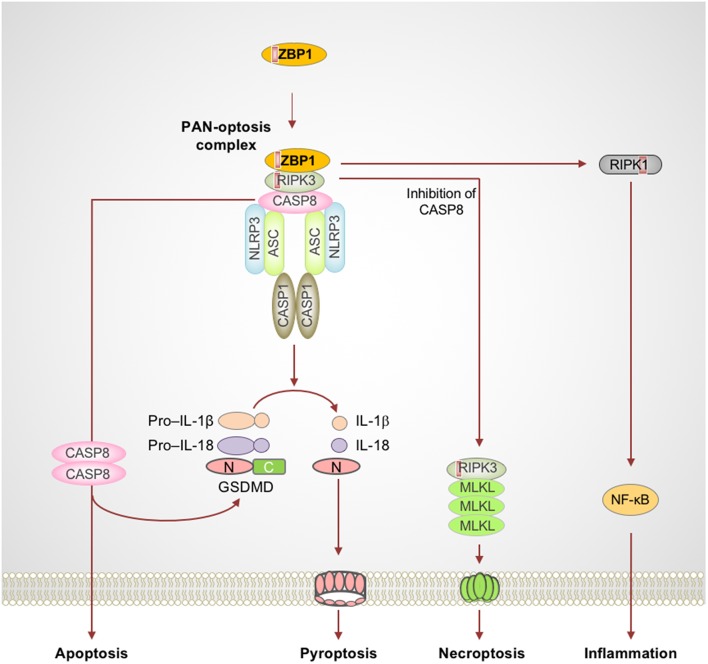
Figure 1.
Activation of ZBP1 triggers assembly of signaling complexes to engage PAN-optosis. Z-DNA binding protein 1 (ZBP1) is an innate immune receptor that senses nucleic acids and activates PAN-optosis and inflammation. ZBP1 activation leads to its interaction with receptor-interacting serine/threonine-protein kinase 3 (RIPK3) and recruitment of caspase-8 (CASP8) to form cell death signaling scaffolds. This ZBP1-RIPK3-CASP8 complex engages nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome-dependent pyroptosis, CASP8-mediated apoptosis, and RIPK3-mixed lineage kinase domain-like pseudokinase (MLKL)–driven necroptosis. ZBP1 also induces RIPK1-driven NF-κB activation and inflammation in response to influenza infection. Red boxes within proteins represent the RIP homotypic interaction motif (RHIM) domain. ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; C, C-terminus; CASP1, caspase-1; GSDMD, gasdermin D; N, N-terminus.