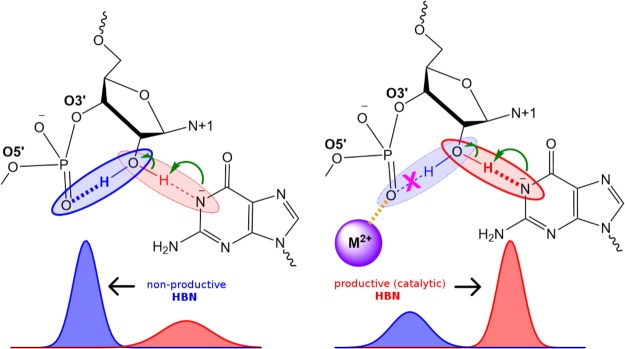
Figure 1.
Tertiary γ (HBN) catalysis induced by metal ion binding. Schematic illustrating 3°γ (HBN) catalysis induced by a divalent metal ion. In the absence of any metal ions (left) the HO2′ can form a nonproductive interaction with a NPO of the scissile phosphate that competes with productive hydrogen bonding with the Watson–Crick edge of the general base guanine in deprotonated (activated) form. In the presence of a metal ion interacting with that NPO (right), the nonproductive interaction is weakened and consequently productive interactions are promoted in which the HO2′ hydrogen bonds to the N1 position of the deprotonated general base guanine as required for nucleophile activation. Here “HBN” is used to distinguish a specific form of 3°γ catalysis that more generally involves the promotion of nucleophile activation through modification of the structural scaffold or hydrogen bond network that organizes the enzyme active site.