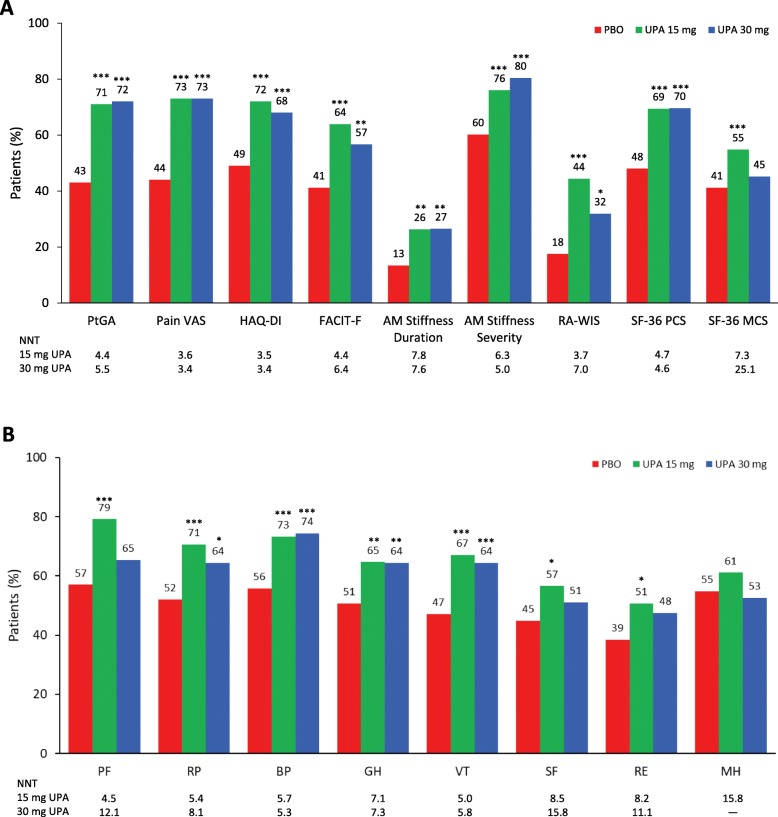
Fig. 2.
Percentage of patients reporting improvements ≥ MCID at week 12. a Patient’s Global Assessment of Disease Activity (PtGA), pain, Health Assessment Questionnaire-Disability Index (HAQ-DI), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), morning (AM) joint stiffness duration, AM stiffness severity, Work Instability Scale for RA (RA-WIS), and Short Form 36 Health Survey (SF-36). b SF-36 individual domains. Baseline values and SF-36 domains were re-scored from 0 to 100. ***P < 0.001, **P < 0.01, *P < 0.5 for upadacitinib versus placebo. BP, Bodily Pain; GH, General Health; MCID, minimum clinically important difference; MCS, mental component summary; MH, Mental Health; NNT, number needed to treat; PBO, placebo; PCS, physical component summary; PF, Physical Functioning; RE, Role-Emotional; RP, Role-Physical; SF, Social Functioning; UPA, upadacitinib; VAS, visual analogue scale; VT, Vitality