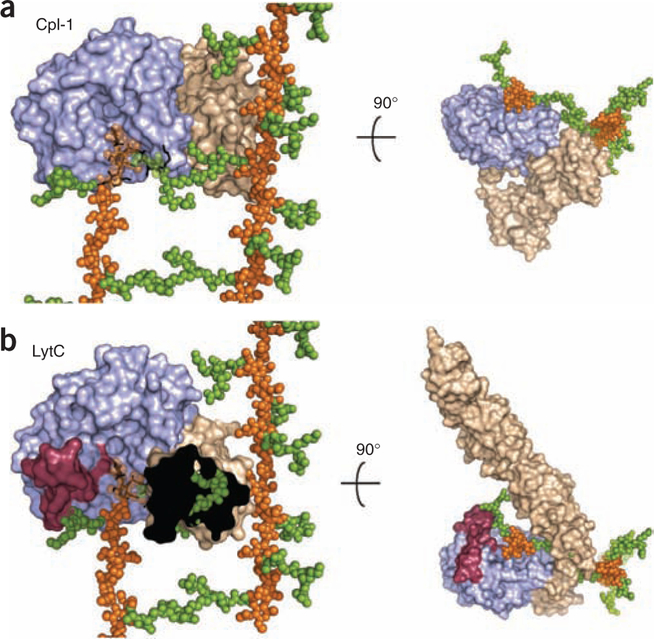
Figure 4.
Superimposition of LytC–choline–PG and Cpl-1–(2S5P)2 crystallographic complexes onto larger PG framework as deduced by its NMR structure22. (a) Molecular surface of Cpl-1 catalytic module is colored in light blue and the CBM in light brown. PG model as determined by NMR is drawn with glycan chains as orange spheres and peptide-stems as green spheres. The (2S5P)2 PG analog bound to Cpl-1 (ref. 19) (PDB 2J8G) is drawn as black sticks. In the superimposed region NMR glycan chains are semitransparent for clarity. (b) LytC molecular surface is represented by modules colored as in panel a. Catalytic modules of Cpl-1 and LytC are in the same orientation, with the PG model superimposed as in panel a. PG fragment bound to LytC is drawn as black sticks. Left, CBM of LytC is not fully depicted (the black area) to appreciate steric encumbrance with peptide stems of PG that would exist in a crosslinked arrangement. The presence of the cross-linked peptide would abrogate binding.