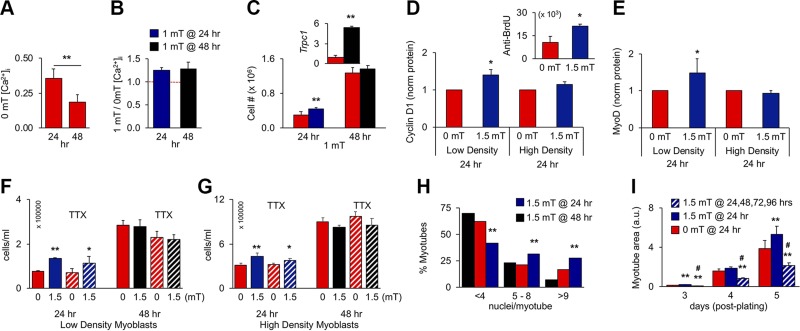
Figure 3.
PEMF exposure enhances in vitro myogenesis when applied once before 24 h postplating. A) Resting [Ca2+]i at 24 or 48 h in culture; n = 4. B) PEMF-induced [Ca2+]i at 24 or 48 h; n = 4. Despite resting [Ca2+]i being depressed at 48 h (A), PEMFs remained capable of augmenting [Ca2+]i (B), indicating that TRPC1-mediated calcium entry was operational, albeit down-regulated at 48 h. C) Proliferative responses to 1 mT exposure at 24 or 48 h; n = 8 (inset: despite the absence of proliferative response at 48 h, Trpc1 transcript levels rose 1 h after exposure). D, E) Cyclin D1 (D) and MyoD (E) protein levels following PEMF exposure at 24 h on low-density (∼2500 cells/cm2) or high-density (∼8500 cells/cm2) cultures; n = 3 or 5, respectively. Protein was collected 24 h after PEMF exposure as indicated. Inset: anti-BrdU-FITC positive myoblasts 6 h after PEMF exposure. F, G) Effects of TTX (1 µM) on PEMF-mediated proliferation applied at 24 and 48 h, as indicated, in low-density (F) or high-density (G) myoblast cultures. H) Nuclei/myotube distribution for exposures to 1.5 mT at 24 (blue) or 48 h (black); 0 mT (red). *P < 0.05, **P < 0.01 (with regard to 0 mT, respectively) (A–G). I) Effect of repeated stimulation on differentiation on myoblast cultures exposed once (blue; 24 h) or consecutively for 4 d (hatched). Myoblasts cultures were exposed at the indicated times and then either analyzed for proliferation/calcium entry (A–G) or induced to differentiate for subsequent analysis (H–I). **P < 0.01, #P < 0.05 with regard to 0 or 1.5 mT, respectively (I). All PEMF exposures were 10 min.