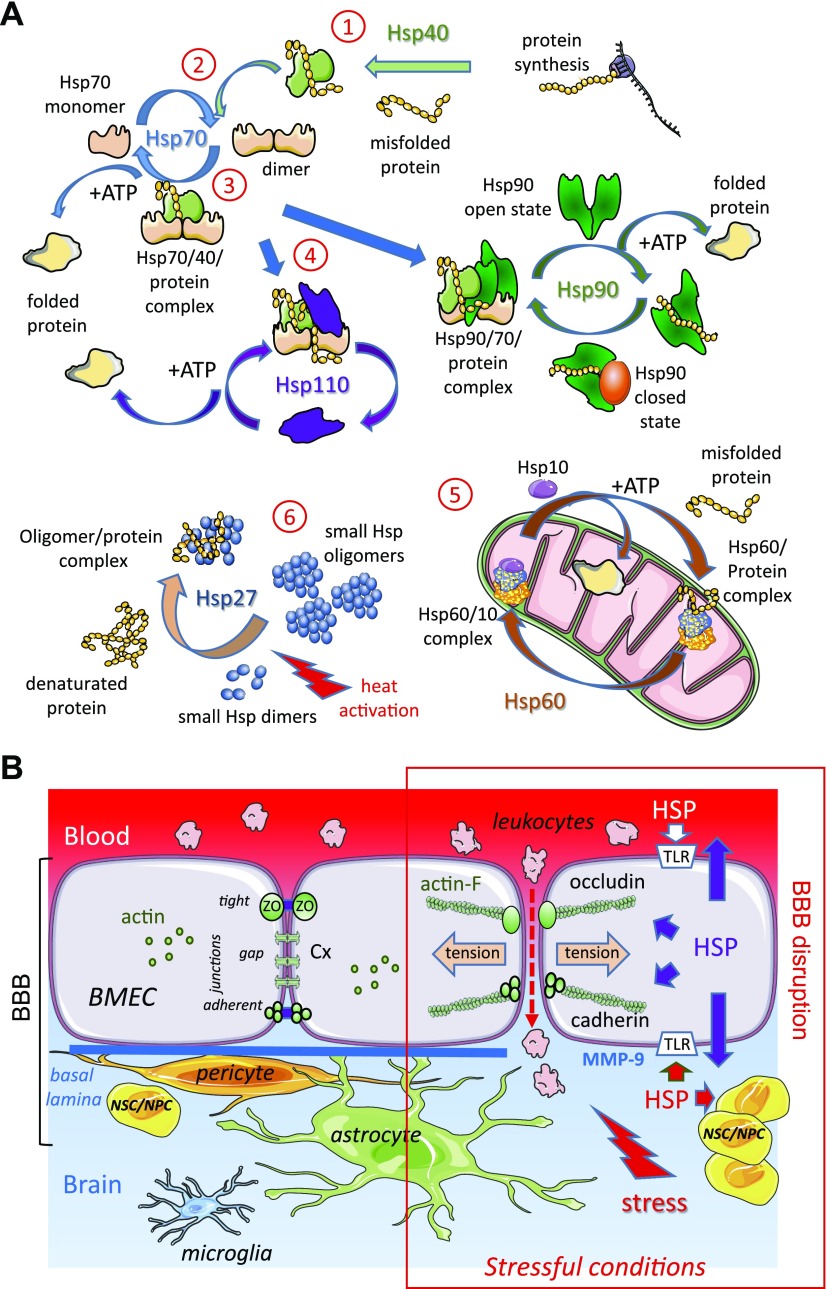
Figure 1.
The HSP chaperone system at the BBB level. A) Collaboration between HSPs in protein folding. Under nonstressful conditions, HSPs bind to newly synthesized polypeptides and facilitate their folding (folded protein), whereas under stressful conditions they recognize denatured proteins and prevent their premature degradation. 1) Hsp40 transiently associates with an altered or misfolded protein to deliver it to Hsp70. 2) Hsp40 binds to Hsp70 and promotes its ATPase activity, thereby generating ADP-linked Hsp70, which stably interacts with the misfolded protein. After nucleotide exchange (replacement of ADP with ATP), the protein is released from Hsp70 and leaves the chaperone system as a correctly folded protein. 3) During stress, Hsp70, in its ADP binding form, encompasses denatured protein. 4) The renaturation of the protein by Hsp70 requires the participation of cochaperones such as Hsp110 or Hsp90. In all cases, the replacement of ADP with ATP is necessary to release the correctly folded protein from these HSP complexes. 5) Some proteins require a subsequent folding step by a group of chaperones having a common structure, chaperonins: for example, Hsp60/Hsp10 present in mitochondria. 6) Under conditions where the system is saturated by a massive influx of proteins to replicate, a transient storage of these is carried out by oligomeric structures formed by Hsp27. These reservoirs of folding intermediates would then direct the proteins to the different protein steps of folding machinery. B) Interactions between astrocytes, pericytes, BMECs, and NSCs/NPCs ensure adequate cerebral blood flow. BMECs are tightly connected through transmembrane proteins present in the tight junctions (occludin), gap junctions (connexin, Cx), and adherens junctions (cadherin). These junction structures interact with cytosolic adaptor proteins that link junctions to the actin cytoskeleton, leading dynamic structures that respond to the local microenvironment. In stressful conditions, HSPs are overexpressed in and released by all cells in the brain. HSPs modulate the expression and/or activity of MMPs involved in basal lamina degradation; for example, MMP-9, which contributes to the disruption of BBB causing cerebral hemorrhage, is decreased by Hsp70 but increased by Hsp90α. Extracellular HSPs bind to TLRs and stimulate specific signaling pathways in the different cell types. In BMEC, these events result in actin polymerization (actin-F), actomyosin contraction (tension), junctional remodeling, and disruption of intercellular contacts, leading to BBB permeability increase. They also signal to adult niches of NSCs/NPCs to increase proliferation and differentiation in healthy or diseased cells. These processes allow transendothelial migration of leukocytes and can be induced by leukocyte secretions themselves. For example, Hsp70 released by M2 monocytes disrupts interendothelial communication by decreasing endothelial expression of Cx43 (67).