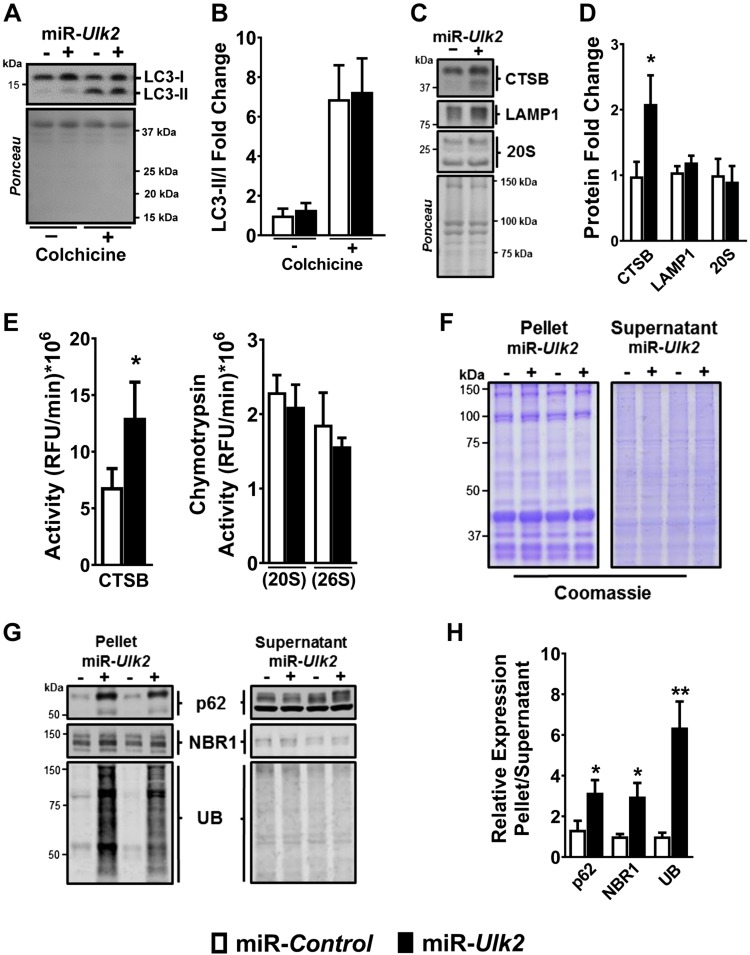
Figure 4.
ULK2 deficiency causes deposition of insoluble ubiquitinated protein aggregates without impairing autophagy or the proteasome. Data were obtained from control and ULK-deficient TA muscles 1 wk after electroporation. A) Representative immunoblots of LC3-I and LC3-II in vehicle (−)– or colchicine (+)–treated control and ULK2-deficient muscles. B) Quantification of LC3-II/LC3-I immunoblot (n = 5). C) Representative immunoblots of CTSB, LAMP1, and 20S proteasomal subunit proteins in control and ULK2-deficient muscles. D) Quantification of CTSB, LAMP1, and 20S in control and ULK2-deficient muscle (n = 5–7). E) CTSB activity (left) and chymotrypsin activity (ATP-independent [20S] and ATP-dependent [26S]) (right) in control and ULK2-deficient muscles (n = 6). F) Representative Coomassie Blue stain of pellet and supernatant fractions of control and ULK2-deficient muscles. G) Representative immunoblots of adaptor and ubiquitinated proteins in the pellet and supernatant fractions of control and ULK2-deficient muscles. H) Quantification of adaptor and ubiquitinated proteins in the pellet and supernatant fractions (n = 7). UB, ubiquitin. Data are means ± sem. *P < 0.05, **P < 0.01.