Figure 6.
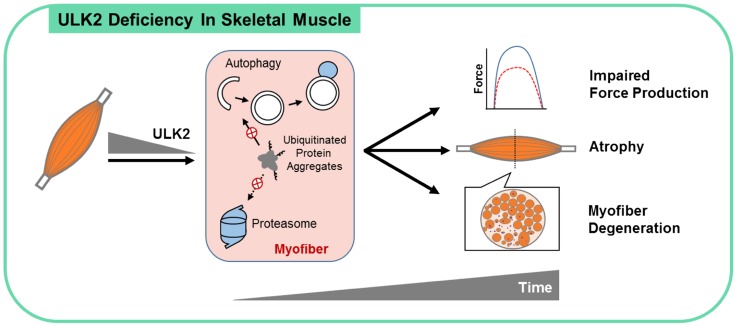
ULK2 is essential for skeletal muscle homeostasis. Our studies reveal that the Ulk2 gene presents a skeletal muscle–enriched pattern of expression in mice and that its deficiency in skeletal muscle, despite not impairing autophagy flux and proteolytic activities of the lysosome and proteasome, leads to robust accumulation of insoluble ubiquitinated protein aggregates associated with the adaptors p62 and NBR1. These findings suggest a key role for ULK2 in modulating the recognition and sequestration of ubiquitinated protein aggregates for degradation by autophagy (and potentially by the proteasome). The ensuing inability of ULK2-deficient muscle fibers to clear proteotoxic aggregates leads to atrophy, impaired force production, myofiber degeneration, and a generally unhealthy morphology of the muscle. Of note, these cellular events and functional outcomes are not observed in ULK1-deficient muscle. Autophagy is depicted with a phagophore and an autophagosome (white) and lysosome (blue).