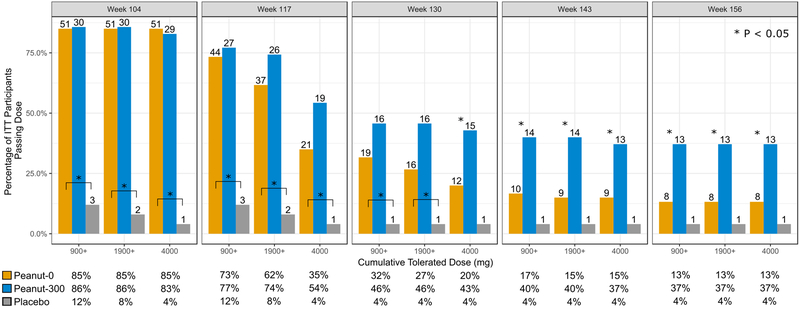
Figure 2.
Percentage of participants who tolerated cumulative peanut challenge dose of 900 mg, 1900 mg and 4 g by DBPCFC week and treatment arm for the ITT population. Number on the top of each bar is the number of participants. P-values based on Fisher’s exact test between peanut-0 (orange bar) and peanut-300 (blue bar), or, highlighted by brackets, comparisons between placebo and peanut-0. Although not noted in the figure, all comparisons between placebo and peanut-300 had P < 0.05. Further detailed percentages are provided below each panel. Peanut-0 = oral immunotherapy with peanut discontinuation arm; Peanut-300 = oral immunotherapy with peanut continuation arm (300 mg).