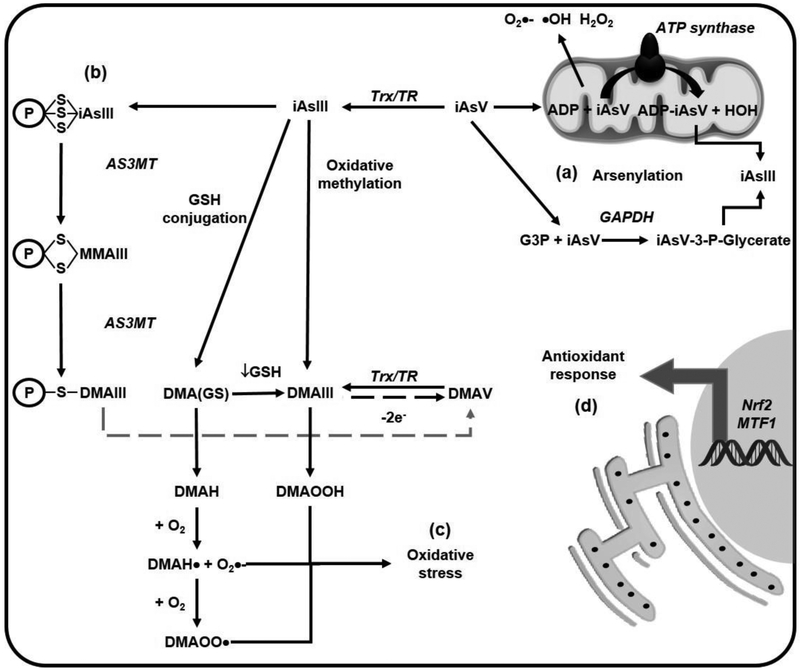
Figure 2. Alterations in mitochondrial function and redox balance induced by iAs.
(a) Arsenate (AsV) can replace phosphate in several metabolic pathways (arsenylation) where the end product is the reduction of AsV to arsenite (AsIII). AsV uncouples oxidative phosphorylation and ATP formation in the mitochondria by binding to ADP via the ATP-synthase. AsV is also arsenylated by glyceraldehyde 3-phosphate dehydrogenase (GAPDH) to generate 1-arsenato-3-phospho-D-glycerate (iAsV-3-P-glycerate) from glyceraldehyde 3-phosphate (G3P). (b) iAsIII binds to protein thiols (coenzyme A, lipoic acid and zinc finger domain containing proteins such as metallothionenin [MT]) and is methylated while still conjugated to these proteins, (c) Dimethylarsinic GSH [DMA(GS)] can form dimethylarsine (DMAH) and react with molecular oxygen (O2) to form DMAH radical (DMAH•) and the DMAH peroxyl radical (DMAOO•). Dimethylarsinous acid (DMAIII) reacts with O2 and forms dimethylated arsenic peroxide (DMAOOH). These peroxyl radicals lead to lipid peroxidation and protein carbonylation (oxidative stress), (d) AsIII induces an antioxidant response via nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and metal-responsive transcription factor-1 (MTF1).