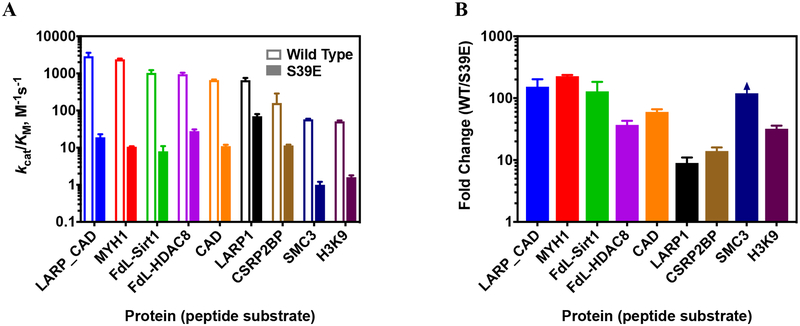
Figure 4. Comparison of S39E and wild-type HDAC8 deacetylation of peptides.
A. Catalytic efficiencies, kcat/KM, of wild-type and S39E HDAC8-catalyzed deacetyation of peptides listed in Table 2 as measured by the Fluor de Lys assay (FdL-HDAC8 and FdL-Sirt1 peptides) and the acetate assay (remaining peptides). B. fold change in catalytic efficiency of S39E HDAC8 compared to wild type for peptides. For all three graphs, peptides are ordered from most to least active with wild-type HDAC8. Error bars are shown in same colors as columns. Substrate names on X-axis correspond to peptides listed in Table 2.