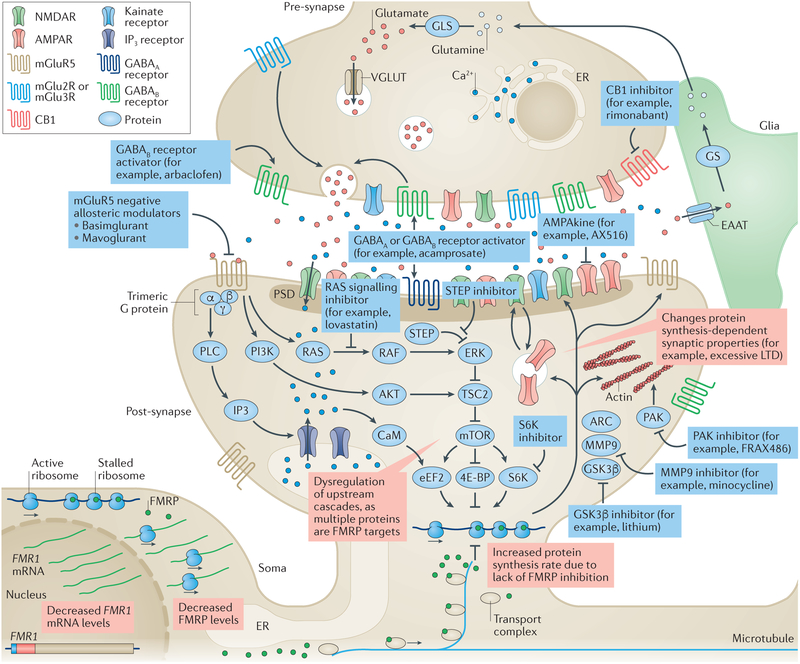
Figure 1 |. Drug targets in fragile X syndrome under investigation.
Glutamate activates a range of ionotropic and metabotropic receptors, including metabotropic glutamate receptor 5 (mGluR5). Activation of mGluR5 leads to activation of Gαq/o and formation of IP3 via phospholipase C (PLC) and intracellular Ca2+ mobilization. mGluR5 also acts (among other effects) on the phosphoinositide 3-kinase (PI3K)–AKT and RAS–ERK pathways, thereby increasing mTOR and ribosomal protein S6 kinase (S6K) activity, ultimately modulating protein synthesis, which is key for regulating synaptic strength. mGluR5 also interacts with NMDA receptors (NMDARs) by way of phosphorylation and receptor trafficking, and with α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (AMPARs) by modulating membrane insertion and receptor subunit composition. Fragile X mental retardation protein 1 (FMRP) is an RNA-binding protein that regulates protein biosynthesis by inhibiting mRNA translation through ribosomal stalling. In patients with fragile X syndrome (FXS) (red boxes), an increase in CGG repeat length and subsequent hypermethylation of FMR1 result in reduced FMR1 transcript levels and decrease FMRP levels. The lack of FMRP at the synapse leads to an increased rate of protein synthesis of FMRP targets. These changes alter downstream protein synthesis-dependent processes, causing long-term depression (LTD), probably due to increased AMPA receptor exocytosis. Blue boxes represent interventions under consideration. mGlu5 negative allosteric modulators can correct multiple aspects of the molecular pathophysiology, including increased phosphorylation of S6K and mTOR, and the rate of protein biosynthesis. AMPAkines can counterbalance the increased AMPAR internalization, enhancing AMPAR sensitivity to glutamate. GABAB receptor activators inhibit glutamate release into the synaptic cleft, which in turn reduces activation of mGluR5 and other glutamate receptors. RAS–ERK signalling inhibitors target the downstream cascades of mGluR5. Genetic reduction of striatal-enriched protein-tyrosine phosphatase (STEP) levels can correct multiple phenotypes in Fmr1-knockout (KO) mice133. Glycogen synthase kinase 3β (GSK3β) is a key target of lithium, which can ameliorate multiple phenotypes in Fmr1-KO mice (see REF. 129). Matrix metalloproteinase 9 (MMP9) is upregulated in FXS, and MMP9 inhibitors correct multiple Fmr1-KO phenotypes126. p21-activated kinase (PAK) is a small G protein that modulates actin dynamics, and PAK inhibitors can revert multiple phenotypes in Fmr1-KO mice53. S6K is essential for regulating cellular protein synthesis, and genetic reduction49 or pharmacological inhibition136 of S6K1 corrected multiple phenotypes in Fmr1-KO mice. Increased cannabinoid receptor 1 (CB1)-mediated signalling has been reported in Fmr1-KO mice, and administration of rimonabant corrected several phenotypes in these mice52,138. Acamprosate, which activates GABAB and GABAA receptors, also ameliorated several phenotypes in Fmr1-KO mice. A more detailed discussion of novel drug targets for FXS is covered in recent reviews61,148,149. 4E-BP, eukaryotic translation initiation factor 4E-binding protein; ARC, activity-regulated cytoskeleton-associated protein; CaM, calmodulin; EAAT, excitatory amino acid transporter; eEF2, elongation factor 2; ER, endoplasmic reticulum; GLS, glutaminase; GS, glutamine synthetase; PSD, postsynaptic density protein; TSC2, tuberin; VGLUT, vesicular glutamate transporter.