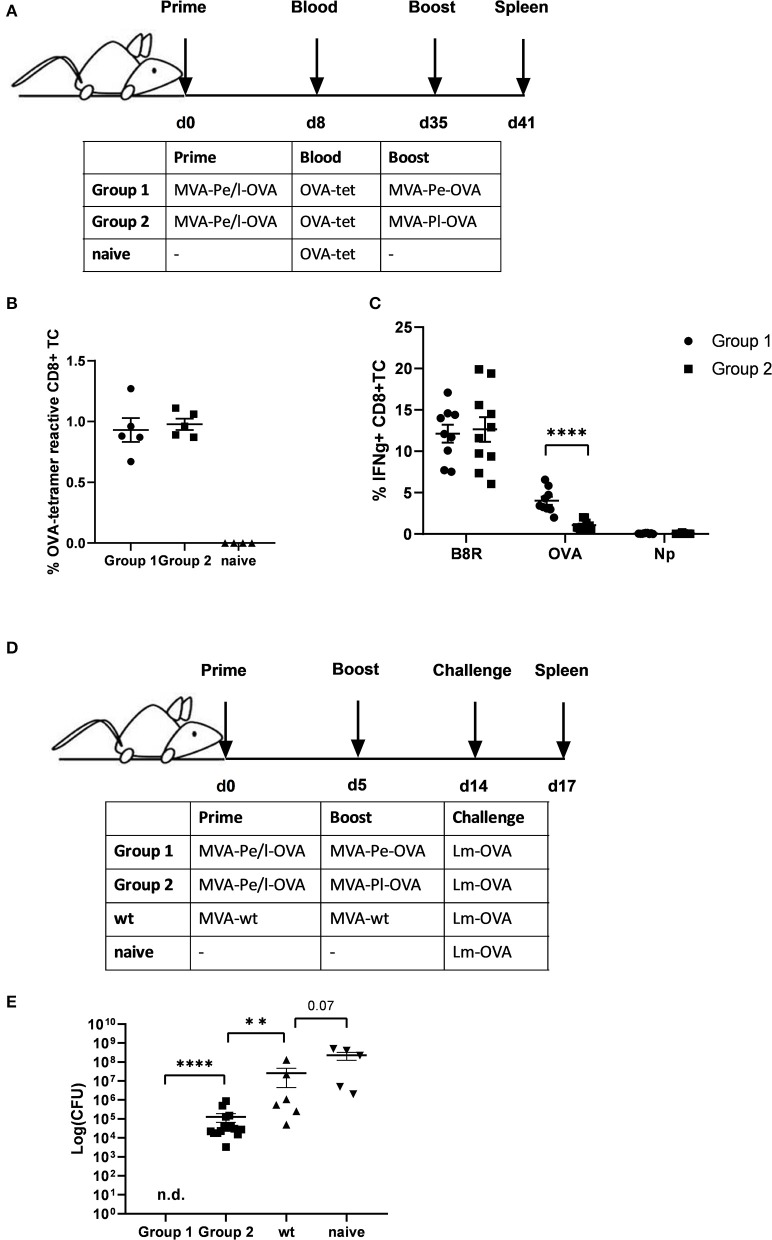
Figure 4.
Reduced CD8+ T cell responses and loss of protection against a pathogen challenge in vivo when immunogenic antigens were expressed late by MVA vectors. (A) Scheme for heterologous MVA-prime-boost vaccination experiment in C57BL/6 mice. For groups 1 and 2, mice were primed i.p. with 108 IU MVA-Pe/l-OVA expressing OVA early and late. On day 8, blood was taken for OVA-specific tetramer staining (results shown in B). On day 35, mice in group 1 were boosted with 108 IU MVA-Pe-OVA (expressing OVA early) and in group 2 boosted with 108 IU MVA-Pl-OVA (expressing OVA late). On day 41, spleens were taken and CD8+ T cell responses analyzed (results shown in C). (B) Priming. OVA275-specific tetramer staining of PBMC from mice primed with MVA-Pe/l-OVA (group 1 and 2) at 8 d p.i. or from uninfected mice (naive), demonstrated comparable OVA275-specific T cell frequencies in group 1 and 2 before boosting. (C) Boosting. On day 35 (memory phase), group 1 was boosted with MVA-Pe-OVA; group 2 with MVA-Pl-OVA. Six days later, ICS and FACS analysis was performed determining B8R- and OVA-specific IFNg production in splenocytes from both groups. Influenza nucleoprotein peptide (Np) served as negative control. (D) Scheme for bacterial challenge after heterologous MVA-prime-boost in vivo. Mice were primed on day 0 and boosted on day 5 with 108 IU recMVA (group 1 and 2) or MVA-wt, or were left unvaccinated (naïve). Nine days later (day 14) all mice were challenged i.v. with 2 × 106 CFU Lm-OVA. (E) Three days after the challenge (d17), the residual bacterial loads were determined as colony forming units (CFU) in the spleen. n.d. indicates not detectable. All data are means and SEM from three independent experiments. Each dot represents one mouse. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 (two-tailed Student's t-test).