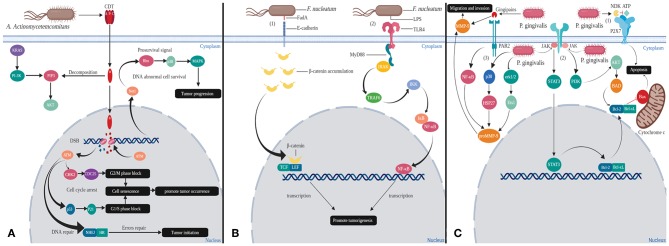
Figure 1.
Mechanisms of oral bacteria virulence factors inducing changes in host cells. (A) Cytolethal distending toxins (CDT) are the virulence factors released by A. actinomycetemcomitans: In the cytoplasm, the phosphatase activity of CdtB can decompose PIP3, thereby over-activating PI-3K, which is one of effectors of KRAS. This process may cause KRAS mutation that leads to cancer. In the nucleus, CdtB causes double strand break (DSB), which activates ataxia telangiectasia mutated (ATM) kinase. Activation of ATM kinase blocks G1/S and G2/M phases promoting tumor occurrence through cell senescence. Tumor initiation also could occur in the instance of erroneous in homologous recombination (HR) and non-homologous end joining (NHEJ) repair mechanisms. In order for the cells to survive, RhoA and p38 MAPK will get activated, thereby promoting tumorigenesis. (B) FadA and LPS are the significant virulence factors of F. nucleatum. (1) Binding of FadA to host cell E-cadherin causes accumulation of β-catenin in cytoplasm that eventually enters into nucleus. β-catenin will act together with LEF/TCF and produce abnormal proteins, which ultimately leads to cancer. (2) LPS binds to host cell TLR4 receptor and induces MyD88 recruitment. These will activate NF-κB signaling pathway to direct cell proliferation and cancer development. (C) Gingipains and NDK are the virulence factors secreted by P. gingivalis. (1) NDK can decompose ATP and inhibit p2x7-mediated apoptosis. (2) Gingipains able to upregulate matrix metalloproteinase 9 (MMP-9) outside the cells and proMMP-9 via NF-kB pathways in the cytoplasm that contribute to the metastasis of cancer cells. (3) P. gingivalis also could enter the cells and increase the expression of proMMP-9 by activating erk1/2-ets1, p38/HSP27. Moreover, P. gingivalis invasion could inhibit release of cytochrome c and activate caspase-9 and caspase-3 by dual JAK/Stat and Akt signaling, thereby allowing damaged or diseased cells to survive.