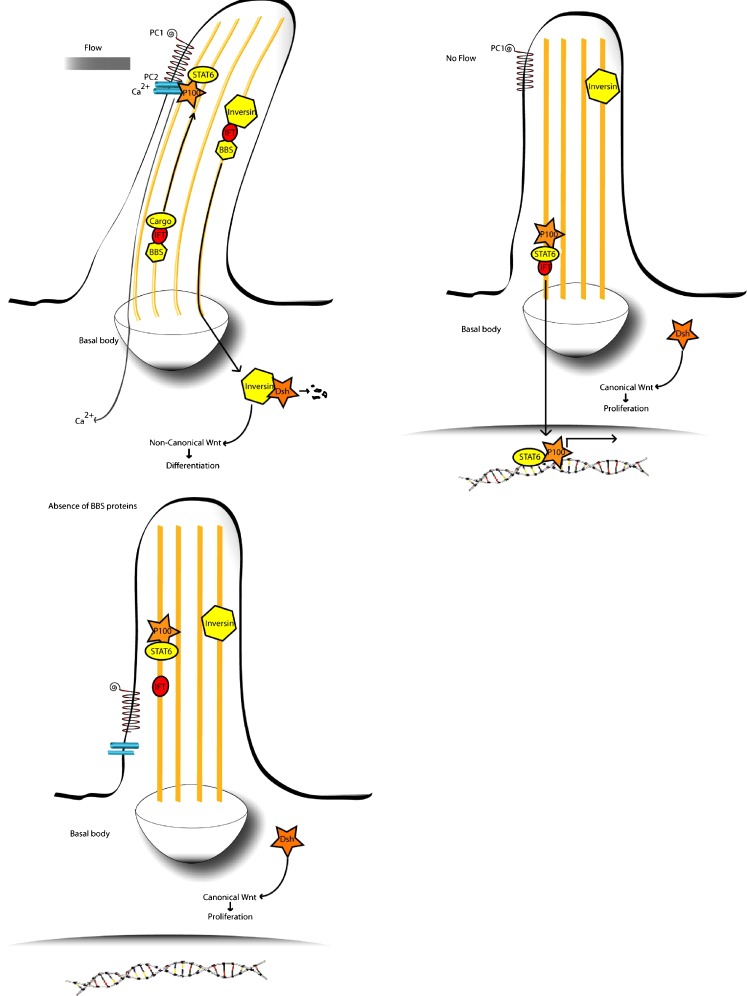
Fig. 1.
Putative pathomechanism for renal cystic hyperplasia in BBS. Top left Urine flow through the kidney tubule causes an influx of Ca2+ ions through polycystin 2 (PC2), while polycystin 1 (PC1) anchors the transcriptional complex of P100 and STAT6 in the cilium. Concurrently, inversin is translocated to the nucleus, where it targets cytoplasmic Dishevelled for destruction, activating the non-canonical pathway and causing cells to differentiate. The ciliary localisation of PC1/2 and inversin may be dependent on BBS proteins. Top right Absence of urine flow reduces Ca2+ influx and causes release of P100 and STAT6, allowing them to enter the nucleus to activate transcription. It also prevents translocation of inversin to the cytoplasm, maintaining the cytoplasmic pool of Dishevelled, which activates the canonical Wnt pathway and causes proliferation. Bottom left A lack of BBS protein function may inhibit proper transport of PC1/2 to the distal tip of the cilium and also prevent translocation of inversin to the cytoplasm. This would cause inappropriate activation of STAT6 target and maintenance of cytoplasmic Dishevelled, leading to unregulated cell proliferation. Additionally, lack of BBS protein function could disturb planar cell polarity (PCP). The combination of disorganised cell polarity and cell division could cause the various abnormalities seen in BBS