Summary
During cytokinesis, animal and fungal cells form a membrane furrow via actomyosin ring constriction. Our understanding of actomyosin ring-driven cytokinesis stems extensively from the fission yeast model system. However, unlike animal cells, actomyosin ring constriction occurs simultaneously with septum formation in fungi. While the formation of an actomyosin ring is essential for cytokinesis in fission yeast, proper furrow formation also requires septum deposition. The molecular mechanisms of spatiotemporal coordination of septum deposition with actomyosin ring constriction are poorly understood. Although, the role of the actomyosin ring as a mechanical structure driving furrow formation is better understood, its role as a spatiotemporal landmark for septum deposition is not widely discussed. Here we review and discuss recent advances describing how the actomyosin ring spatiotemporally regulates membrane traffic to promote septum driven cytokinesis in fission yeast. Finally, we explore emerging questions in cytokinesis, and discuss the role of extracellular matrix during cytokinesis in other organisms.
Keywords: actomyosin ring, septum, yeast, membrane trafficking, constriction, cleavage furrow
Graphical Abstract
Cytokinesis in fission yeast involves actomyosin ring constriction that occurs simultaneously with septum (cell wall) synthesis. In this review we discuss how these two processes are coordinated and how multiple membrane trafficking events are necessary for proper cytokinesis.
Introduction
Cytokinesis, the final step in cell division, is essential for growth, development, and cell differentiation [1, 2]. After the nucleus divides by mitosis, the cell splits its cytoplasm into two via cytokinesis, giving rise to two daughter cells. Successful cytokinesis ensures that the two daughter cells maintain genomic integrity and chromosome number during division. In animal and fungal cells, cytokinesis occurs via the constriction of an actomyosin based contractile ring to form the cleavage furrow [3]. The fission yeast, Schizosaccharomyces pombe, is an excellent model system for understanding the molecular mechanism for actomyosin ring formation [4]. However, cytokinesis in fission yeast is more complex as it also requires the formation of a septum (cell wall) at the division site in addition to assembling a contractile actomyosin ring. Upon mitotic commitment, the cell prepares for cytokinesis through the recruitment of proteins necessary for ring formation to the precursor nodes [5, 6]. The Septation Initiation Network (SIN) pathway, activated by the GTPase Spg1, promotes proper positioning of the precursor nodes to the cell middle surrounding the nucleus to ensure that the actomyosin ring forms between the incipient daughter nuclei, thus preventing polyploidy and aneuploidy [4, 7] . This ensures that the cell divides along the middle, thereby generating two equal sized daughter cells.
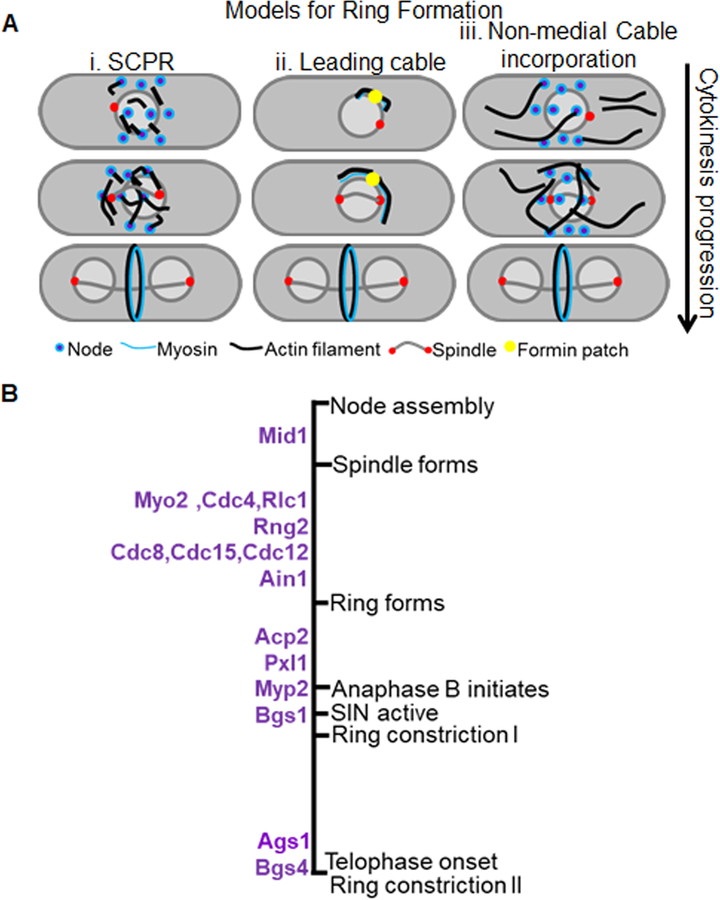
The proteins that are recruited to the precursor nodes prior to initiation of cytokinesis, and their dynamics, have been assiduously described in several studies [5, 6, 8–11]. Once the precursor nodes recruit the formin Cdc12, actin nucleation and filament formation occur [5, 12, 13]. Different models have addressed how the actomyosin ring is assembled during cytokinesis; one model proposes that the type II myosin interacts with actin filaments using a search, capture, pull, and release mechanism to form a ring (Figure 1Ai) [8]. An alternate model, that is not mutually exclusive, proposes that the ring assembles from a single site, where two groups of parallel actin filaments of opposing direction rearrange to form a structure with mixed directionality (Figure 1Aii) [14]. A third model proposes that non-medial actin cables generated mainly by the formins Cdc12 and to an extent For3 are incorporated into the cells medial region to condense into a ring (Figure 1Aiii) [15, 16]. The SIN pathway works in parallel with the spindle assembly checkpoint to ensure that constriction initiates only after initiation of chromosome segregation [11, 17–21]. Unlike most mammalian cells where ring constriction initiates immediately upon ring assembly, in fission yeast the ring undergoes a maturation or dwell phase following its assembly in early anaphase A [22]. During anaphase B, the maturation phase ends, and ring constriction is initiated [11]. A recent report indicates that ring constriction occurs in two phases: a slow phase that initiates during anaphase B and a faster phase once anaphase completes [11, 23],. The constriction of the ring is concurrent with septum deposition along the membrane adjacent to the ring [11, 24]. The septum, once built, physically separates the two daughter cells. It is comprised of a middle layer (primary septum) flanked by two secondary septa [25–27]. Several reviews extensively describe the molecular structure of the fission yeast septa [28, 29]. After completion of ring constriction, the septum matures, and the middle layer (primary septum) is degraded by digestive enzymes, marking the completion of cytokinesis [30, 31]. The secondary septum remains intact and serves as the new cell wall for the two daughter cells [27]. This process of septum synthesis during furrow formation is unclear and the molecular details of its spatiotemporal coordination with actomyosin ring constriction are poorly understood.
Figure 1. Early cytokinetic events.
A. Models for actomyosin ring assembly. i. The search-capture-pull-release model- the formin Cdc12 when recruited to the nodes nucleates actin while the type 2 myosin binds actin and pulls on the filaments to coalesce into a ring. ii. The Leading cable model- A patch of formin, Cdc12, nucleates actin cables that extends along the periphery of the division site to eventually form a ring. iii. Non-medial cable incorporation model. The formins Cdc12 and For3 nucleate actin to form non-medial cables that are then incorporated into the cells medial region to for a ring. B. Timeline of key proteins recruited to the division site leading to ring constriction with reference to different cytokinetic steps and mitotic progression. Biphasic ring constriction is depicted as Ring constriction I that occurs during anaphase B after Bgs1 recruitment and is slow and as Ring constriction II that occurs during telophase after recruitment of Ags1 and Bgs4 and is accelerated.
Studies on the regulation of cytokinesis in fission yeast have focused mostly on the structural aspects of the ring and how it is assembled [24, 32, 33]. Recently, super-resolution microscopy enabled the spatial mapping of proteins localized to the cytokinetic nodes and to the ring after assembly, showing their proximity to the ring and the membrane [34, 35]. However, once the ring assembles, how the organization of these proteins drive concurrent ring constriction and septum ingression remains unclear.
Septum driven cytokinesis in fission yeast.
In fission yeast, cytokinesis involves both ring constriction and simultaneous septum/cell wall ingression. The primary septum is mainly comprised of linear 1,3-β-glucan, polymerized by the β-glucan synthase Bgs1 (Table 1) [26, 36]. The secondary septum is structurally different from the primary septum and primarily consists of 1,6-β-branched 1,3-β-glucan, synthesized by Bgs4 (Table 1) [23, 27, 36, 37]. In addition, the primary and secondary septum also consists of α(1,3)-glucan synthesized by Ags1/Mok1 (Table 1) [38–40]. The glucan synthase homolog Bgs3 also localizes to the septum during cytokinesis and is speculated to contribute to the synthesis of 1,6-β-branched 1,3-β-glucan [41]. Bgs1–3 and Ags1 are integral membrane proteins and localize to the division site in a manner dependent on the actomyosin ring [23, 25, 27, 38, 39, 41–45]. Bgs1 has been shown to require the ring protein Cdc15 for its localization to the division site [43]. In cdc15 mutants, where Bgs1 recruitment to the division site is impaired, the actomyosin ring is not properly anchored to the membrane and has been shown to slide [43, 46]. The integral membrane protein Sbg1 links the actomyosin ring with Bgs1 [47, 48]. It is unclear if Bgs1 enzyme activity is needed for anchoring the ring. While the authors report that addition of the drug Aculeacin A (blocks glucan synthesis) does not lead to ring sliding, Bgs1 is resistant to this drug and thus one cannot conclude if the Bgs1 enzymatic activity is necessary for anchoring the ring [49, 50]. Bgs1 has been reported to cooperate with Paxillin Pxl1 and Cdc15 to maintain ring integrity and to promote septum synthesis [51]. Similar to cdc15 and bgs1 mutants, pxl1 mutants also show ring sliding defects [51]. Paxillin has been shown to recruit the phosphatase calcineurin Ppb1 to the division site, which in turn dephosphorylates Cdc15 and promotes Bgs1-dependent septum synthesis [52]. During anaphase the SIN pathway is activated and Bgs1 is recruited to the division site as the ring completes maturation and initiates constriction [11]. At this stage the rate of ring constriction and septum ingression is slow and only the primary septum with linear 1,3-β-glucan are synthesized [11]. Once anaphase completes, Bgs4 is also recruited to the division site and the rate of ring constriction and septum ingression are accelerated [11]. It is possible that the acceleration in septum ingression is due to that fact that Bgs4 and Ags1 make 1,6-β-branched 1,3-β-glucan and α(1,3)-glucan respectively, that provide support and rigidity to the septum. Synthesis of a more rigid septum could lead to faster membrane invagination and ring constriction since the septum is coupled to the membrane barrier which in turn is associated with the actomyosin ring [27, 43].
Table 1:
Enzymes delivered to the division site during cytokinesis.
| Enzyme | Polysaccharide | Septum structure | Localization depends on | Reference |
|---|---|---|---|---|
| Septum synthesis | ||||
| Ags1/Mok1 | α(1,3)-D-Glucan | Primary septum and secondary septum | Exocyst complex, Pck2 | [38, 39] |
| Bgs1 | Linear β(1,3)-D-Glucan | Primary septum | Sbg1, SIN pathway, Cdc15, Cdc42, Myo52, TRAPP II complex | [25, 26, 43, 45, 47, 51, 88, 89] |
| Bgs4 | Branched β(1,3)-D-Glucan | Primary and secondary septum | Rga7, TRAPP II complex | [23, 87] |
| Septum digesting | ||||
| Agn1, Eng1 | endo-1,3-alpha-glucosidase | Between the base of the primary septum and the outer cell wall | Rho3, Rho4, Exocyst complex, Septin ring | [30, 31, 93, 95–97, 99, 100, 102, 103, 105, 117, 118] |
Bgs1 enzyme activity promotes proper septum ingression and simultaneous ring constriction [26, 51, 53, 54]. Cells with hypomorphic bgs1 or absence of bgs1 assemble a normal ring but show delayed and slow furrow formation with severe defects in septum morphology [26, 51, 53–55]. It is unclear why septum deposition is required for timely initiation of ring constriction. One report suggests that cell-walled organisms such as fission yeast have high internal turgor pressure that antagonizes furrow formation [54]. This high internal turgor pressure is approximated to be ~0.95MPa, similar to the pressure in a racing bike tire [54]. Mathematical estimations suggest that actomyosin ring constriction does not generate sufficient force to overcome this turgor pressure [54]. Efforts to explain how the high internal turgor pressure is overcome during cytokinesis leave open questions. In vitro studies in yeast protoplasts indicate that constriction is dependent on type II myosin and ATP [56, 57]. However, in vivo experiments have shown that cytokinesis proceeds, even when the actomyosin ring is artificially removed using the actin depolymerizing drug Latrunculin- A [54]. In these Latrunculin-A treated cells, septum ingression must complete about 50% of the septum length, prior to removal of the contractile actomyosin ring for a complete septum to form. Interestingly, the rate of septum synthesis is slower in these cells, compared to untreated cells, indicating that the actomyosin ring is still necessary for efficient cytokinesis. Based on these observations the authors propose a model in which septum ingression provides the force required to overcome internal turgor pressure for membrane furrowing [54]. Actomyosin ring constriction defects reported in mutants with the thermo-sensitive bgs1 allele cps1–191 can also be explained by the fact that Bgs1 is required for anchoring the ring to the membrane and for actomyosin ring integrity [43, 51]. Anomalies in ring stability could also lead to constriction defects. Another consideration is that the actomyosin ring may fail to constrict when it is coupled to a rigid septum. Coupling of the septum to the membrane barrier could be detrimental to membrane ingression and ring constriction as the rigid septum cannot be pulled in during this process. In this scenario, the ring would constrict and form a membrane furrow only upon de novo septum deposition and ingression. Thus an alternate model suggests that under normal conditions, building the septum de novo towards the cell interior as the ring constricts, is necessary for septum ingression [58]. This is further supported by the observation that in bgs4 mutants lacking secondary septum, the ring is no longer coupled to the primary septum and the rate of constriction is faster [27]. Moreover, contrary to the model proposed by Proctor et al., [54] this report shows that the ring can constrict even in the absence of septum ingression suggesting that ring constriction per se does not need septum ingression. It is likely that septum ingression is only necessary when the membrane is coupled to the septum [27].
While septum ingression drives furrow formation, the actomyosin ring is required to spatially coordinate septum synthesis. By using microfluidic chambers to manipulate the shape of the actomyosin ring, it is possible to alter the rate of septum ingression [59]. In regions of the ring with increased curvature, the rate of corresponding septum ingression is higher [59]. Moreover, in cells with misshapen actomyosin rings, curvature-dependent septum ingression over time corrects the shape of the cleavage furrow to a regular circular shape [59]. Thus, it is possible that septum ingression is activated by the actomyosin ring in a mechanosensitive manner. Evidence for mechanosensitive activation of septum synthesis has also been reported by another study [60]. A mathematical model was developed to explain how septum synthesis is spatially coordinated such that the shape of the furrow is fairly circular [60]. This model suggests that septum deposition responds to actomyosin ring tension, resulting in faster septum deposition in regions of the ring with high tension due to increased curvature [60]. Thus, throughout constriction, the septum synthesizing apparatus responds to the forces generated by the actomyosin ring and correspondingly synthesizes septum. The rate of furrow formation is set by the rate of septum synthesis, which is imposed by the force generated from the actomyosin ring [60]. This is also supported by the fact that type II myosin mutants, myo2-E1 and myp2Δ show ring constriction defects and corresponding delays in furrow formation suggesting delays in septum ingression [61].
The contractile actomyosin ring also acts as a landmark for the septum synthesizing apparatus.
While the actomyosin ring is necessary to initiate septum synthesis, it is no longer necessary once septum synthesis initiates [62–65]. It has been reported that apart from providing a contractile structure, the ring has an additional and likely critical role in cytokinesis, where it acts as a landmark for the recruitment of the septum synthesizing apparatus (Figure 1B) [23, 25, 38, 39, 44]. During mitosis, the anillin-like protein Mid1 recruits proteins required for actomyosin ring formation to the cytokinetic nodes [6, 66–68]. Mid1 recruits the type II myosin Myo2, its regulatory light chain Rlc1, the myosin II light chain Cdc4 and the IQ-GAP Rng2 in a complex [6]. Mid1 also recruits the F-BAR protein Cdc15 to the nodes. Both these complexes then independently recruit the formin Cdc12 to the division site [6, 13]. Once the formin Cdc12 is recruited to the nodes it starts nucleation of actin filaments [22]. Assembly of the actomyosin ring is regulated at multiple levels. The Septation Initiation Network (SIN), analogous to the Hippo signaling pathway, couples ring constriction and septum formation with chromosome segregation [17, 69, 70]. The terminal SIN kinase, Sid2, phosphorylates Cdc12, thus promoting its F-actin bundling activity [18]. Thus timely recruitment of the proteins to the nodes and the activation of the SIN pathway ensure that the actomyosin ring starts assembly and is available for constriction once the cells start chromosome segregation [11]. The ring completes assembly during anaphase A but starts constricting only during Anaphase B (Figure 1B) [11, 22]. This delay in constriction could be due to the fact that after its assembly, the ring acts as a landmark to recruit proteins required for septum formation, and constriction only initiates once these proteins are available to start septum synthesis. Indeed, during anaphase B ring constriction is slow when Bgs1 is the only septum synthesizing enzyme recruited (Figure 1B) [11]. Upon telophase onset, Bgs4 is recruited and constriction occurs at a faster rate (Figure 1B) [11]. A key protein involved in recruiting or stabilizing proteins at the actomyosin ring is the F-BAR domain containing protein Cdc15 [42, 46]. The BAR domain of Cdc15 interacts with the membrane, while the SH3 (Src homology 3) domain binds proteins that associate with the ring [42, 46, 71]. Thus Cdc15 is required for anchoring the actomyosin ring to the membrane [34, 43]. The SIN pathway kinase Sid2 also phosphorylates Cdc14, the Clp1 phosphatase which dephosphorylates Cdc15 [19–21]. Phospho-regulation of Cdc15 results in a conformational change that promotes its oligomerization and enables it to scaffold numerous proteins at the interface between the plasma membrane and the actomyosin ring [19]. While Cdc15 is required for cytokinesis, it is not essential for actomyosin ring formation in the presence of an active SIN pathway as observed in cdc15Δ and cdc15–140 mutants [72, 73]. However, actomyosin rings are highly unstable and disintegrate in cdc15–140 mutants suggesting that it is required for ring maintenance [38, 72, 73]. The actomyosin ring in cells lacking cdc15 may not be competent to undergo constriction [43, 72]. This is due to two factors: (i) misregulation of Cdc12, discussed above, and (ii) failure to recruit the septum synthesizing apparatus [23, 25, 38, 39, 43, 44].
Once the ring assembles, Cdc15 levels at the division site continue to increase [74]. Cdc15 via its SH3 domain scaffolds numerous proteins to the division site [42]. The SH3 domain is also required for the localization of Bgs1 to the actomyosin ring [43, 51]. The catalytic activity of glucan synthases requires activation of the Rho GTPase, Rho1 [37, 75]. Cdc15 interacts with the Rho1 GEF (guanine nucleotide exchange factor) Rgf3 and helps recruit it to the division site after actomyosin ring assembly [42, 76–80]. In addition to Cdc15, Imp2, another F-BAR and SH3 domain containing protein, is involved in scaffolding proteins at the actomyosin ring [42, 46]. Cdc15 and Imp2 SH3 domains function in a redundant manner. The SH3 domains of these two proteins function cooperatively and mutants lacking both the SH3 domains are not viable [46]. In cdc15ΔSH3 imp2ΔSH3 double mutants, analysis of the spores indicate that these mutants display fragmented actomyosin rings and fail cytokinesis [46]. Furthermore, tandem affinity purification of the SH3 domains and subsequent mass spectrometry identified multiple proteins that associate with either Cdc15-SH3 or Imp2-SH3 [42]. Thus, the SH3 domains structurally stabilize the actomyosin ring and function as a scaffold that recruits multiple proteins to the ring after assembly. Interestingly, while Cdc15 localizes to the division site prior to onset of actomyosin ring assembly, the primary septum synthesizing apparatus including Bgs1 and Rgf3 localize to the division site only after the ring is assembled [11, 45, 51, 76, 77]. This ensures that septum synthesis does not initiate before chromosomes start segregation. We have shown along with others that Cdc15 levels at the ring rapidly increase after ring assembly, and it is possible that the Cdc15 population recruited after ring assembly specifically promotes localization of Bgs1 and Rgf3 [42, 74, 81]. Bgs1 localization at the membrane also requires the small GTPase Cdc42 [45, 82]. It has been reported that Cdc42 is activated at the division site as the ring completes assembly [45, 83]. Cdc42 activation at the assembled ring depends on the GEF, Gef1, and mutants lacking gef1 display a delay in Bgs1 localization to the ring [45]. It is possible that while Cdc15 is present at the ring from the onset of ring assembly, Bgs1 is recruited to the ring only upon Gef1-mediated Cdc42 activation in the assembled ring [45]. The septum ingresses centripetally indicating that deposition occurs uniformly along the membrane behind the actomyosin ring and along the ingressing membrane. This indicates that Cdc15 and thus the septum building apparatus is uniformly localized along the actomyosin ring leading to centripetal septum deposition [23, 25, 27, 38, 41, 44, 81].
Another F-BAR domain containing protein, Rga7 has been shown to be required for maintaining actomyosin ring integrity, and for proper septum synthesis in cooperation with Cdc15 and Imp2 [84]. Rga7 is a Rho2 GTPase activating protein (GAP), but the catalytic activity is not required for its role in cytokinesis [84, 85]. Rga7 interacts with Rng10, a coiled-coil protein that enables it to interact with the membrane at the division site and promote ring stability [86]. Rga7 has been shown to specifically promote Bgs4 recruitment to the division site [87]. The integral membrane protein Sbg1 has also been shown to promote Bgs1 localization at the septum [47, 48]. Bgs1 and Sbg1 localization are dependent on each other and Sbg1 associates with both Bgs1 as well as the actomyosin ring components thus linking the two [48].
Membrane trafficking events during cytokinesis
Recent reports indicate that Membrane trafficking events at the division site are essential for cytokinesis [45, 88]. Blocking endocytosis using the Arp2/3 inhibitor CK-666 prevents initiation of septum ingression and cell separation during cytokinesis [81]. Interestingly, CK-666 treatment does not inhibit actomyosin ring formation [81]. This suggests that membrane trafficking events are involved in distinct cytokinetic events. The trafficking events participating in the different cytokinetic stages are discussed below.
Actomyosin ring maturation.
After the ring assembles, the Bgs1 is delivered to the membrane adjacent to the ring via actin-mediated delivery (Figure 2A) [11, 45]. The type V myosins have been shown to be required for this process [89]. Actin-mediated delivery depends on the formin For3 that nucleates actin to form cables [90]. It is not clear if For3 is also required for the delivery of glucan synthases. Mutants of for3 only display minor cytokinetic defects suggesting that redundant delivery mechanisms could function here [16]. However, for3 mutants combined with an activated allele of the formin cdc12 is lethal, but in this case the cells fail to assemble an actomyosin ring [16]. Bgs1 localization is also dependent on the F-BAR Cdc15 and the timely activation of the GTPase Cdc42 [45, 91]. Upon blocking endocytosis by CK-666 treatment, the cells form a ring but fail to recruit Bgs1. In addition, the levels of Cdc15 which typically increases at this stage, remains the same [81]. Similar observations were made with the endocytosis defective arp3-c1 cold sensitive mutant [81]. At the maturing ring, Gef1-mediated Cdc42 activation facilitates recruitment of Bgs1 to the ring [45]. On the other hand, Bgs1 delivery to the ingressing membrane depends on Scd1-mediated Cdc42 activation [45]. While the mechanism by which Cdc42 promotes Bgs1 delivery is not clear, gef1 mutants which do not activate Cdc42 during ring maturation show defects in type V myosin, Myo52 localization to the division site and fewer non-medial actin cables [45]. Therefore, it is possible that Cdc42 regulates actin-mediated delivery of Bgs1.
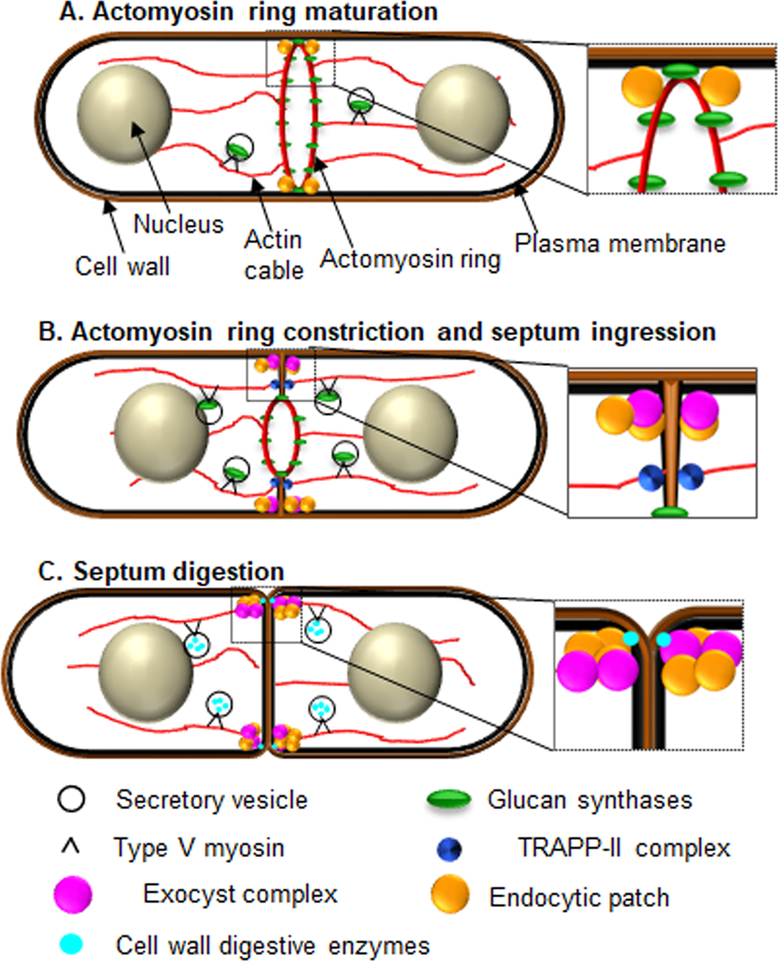
Figure 2: During cytokinesis membrane trafficking events promote septum formation, membrane ingression and septum digestion in fission yeast.
A. Actomyosin ring Maturation: During actomyosin ring maturation the septum synthesizing glucan synthase Bgs1 is delivered to the membrane adjacent to the ring through secretory vesicles and type V myosin, and via the TRAPP-II complex. B. Actomyosin ring constriction and septum ingression: Ring constriction occurs in a biphasic manner. In Anaphase B the actomyosin ring constriction initiates at a slow rate and this is concurrent with septum ingression. In late anaphase B Ags1 is delivered while Bgs4 is delivered after the completion of Anaphase B. At this stage the rate of constriction and septum ingression accelerates. Glucan synthases delivery and membrane deposition continues during ring constriction and septum ingression. Endocytosis and the exocyst complex is mainly restricted to the rim of the ingressing membrane. C. Septum digestion: Cell wall digestive glucanases are delivered to the base of the primary septum via exocyst-mediated delivery leading to cell separation.
It has been shown that Gef1 mediated Cdc42 activation promotes endocytosis [81]. In gef1 mutants, Cdc15 levels at the endocytic patches are higher and the patch itself displays longer lifetimes [81]. In a gef1Δ mutant combined with an activated allele of cdc12, cdc12Δ503, the cells show defects in septum ingression and do not constrict centripetally [81]. In these gef1Δcdc12Δ503 mutants, the cells display irregular distribution of Cdc15 along the ring such that regions of the ring with increased Cdc15 levels constrict at a faster rate. Since Cdc15 recruitment to the assembled ring requires endocytosis [81] and Cdc15 is involved in endocytosis [92], it is possible that after ring assembly, the cell recruits Cdc15 to the ring mainly from the endocytic patch. In agreement, disruption of endocytosis leads to defects in Cdc15 recruitment resulting in its irregular distribution at the ring [81]. These findings indicate that membrane trafficking events such as endocytosis are required to uniformly organize Cdc15 along the ring thereby leading to centripetal septum ingression and constriction.
Actomyosin Ring Constriction and Septum Ingression.
After ring constriction initiates, glucan synthases such as Bgs4 and Ags1 are recruited to the membrane adjacent to the ring and to the ingressing membrane and rate of ring constriction accelerates (Figure 2B) [11, 23, 38, 39]. While Ags1 is recruited during late Anaphase B, Bgs4 is recruited during telophase [11]. Surprisingly, while Cdc42 is required for Bgs1 recruitment, it does not promote recruitment of Bgs4 [45, 91]. It is unknown if Cdc42 is also required for the delivery of Bgs3 and Ags1. Thus, Cdc42 appears to promote delivery of specific cargoes and may not simply regulate actin cable formation during cytokinesis.
Another component of the membrane trafficking machinery is the TRAPP-II delivery complex which has been shown to participate in cytokinesis [88]. This complex not only delivers glucan synthases to the division site, but during ring constriction, it also provides additional membrane via the delivery of vesicles and tubulovesicular structures [88]. The TRAPP-II complex localizes all along the ingressing furrow and is suggested to be primarily required for delivery of membrane necessary for membrane expansion during this process (Figure 2B) [88].
The organization of the trafficking machinery is spatially regulated during cytokinesis. While the TRAPP-II complex promotes tethering and fusion of vesicles along the entire ingressing membrane, the exocyst contributes to delivery only at the rim of the cleavage furrow [88]. It is possible that this spatial segregation of trafficking apparatus facilitates delivery of specific cargoes to different regions of the cleavage furrow. It has also been reported that endocytosis is mainly confined to the rim of the cleavage furrow [88]. It is not clear whether endocytosis is limited to the rim to ensure unhindered membrane expansion at the leading edge of the cleavage furrow, or whether it is required for the redistribution and/or recycling of proteins as cytokinesis progresses.
Septum digestion and cell separation.
After septum formation, septum digesting glucanases are delivered between the outer edge of the primary septum and the lateral cell wall to properly digest the primary septum and allow cell separation (Figure 2C, Table 1) [30, 31, 93–98]. The delivery of the glucanases are dependent on the exocyst complex [93, 94, 99, 100]. The exocyst complex is essential for viability, however temperature sensitive sec8–1 exocyst mutants and germinated spores of deletion mutants show polarized cell shape, indicating that it is not vital to cell polarity [93, 101]. Similarly, sec8–1 mutants under restrictive conditions display a septum, indicating that the exocyst is dispensable for septum formation [93]. However, these mutants fail to localize the glucanases such as Eng1 and Agn1 to the division site, and as a result do not undergo cell separation [94]. Thus, the primary role of the exocyst complex during cytokinesis is the delivery of glucanases necessary for cell separation.
In addition to the exocyst complex, delivery of the hydrolytic enzymes required for septum digestion also depends on the Rho3/4 GTPases [93, 99, 100, 102]. Rho3 promotes the delivery of secretory vesicles to the delivery site, likely through interaction with the exocyst complex [99]. Interestingly, overexpression of rho3+ but not rho4+ can suppress the separation defects of exocyst mutants [99, 100]. However, overexpression of eng1+ or agn1+ suppresses the rho4Δ multi-septation phenotype [103]. rho4Δ cells display secretory defects as they exhibit reduced levels of Eng1 and Agn1 in cell culture, and fail to localize these enzymes to the septum at elevated temperatures [103]. When exocyst functionality is compromised, Rho3 localization to the division site is impaired [99]. In contrast, Rho4 localization is independent of the exocyst complex but the exocyst subunit Sec8 fails to localize to the division site in rho4 mutants [100]. The Rho GEF, Gef3 also contributes to cell separation, likely via Rho4 activation [102]. Interestingly, cell separation defect is elevated in gef3Δgef1Δ double mutants [102]. It is not clear if Gef1-mediated Cdc42 activity is also required for cell separation [102].
Septin protein complexes are also required for the delivery of glucanases to the division site. This includes the anillin homolog Mid2 and the septin proteins Spn1–4 [94, 98, 100, 102, 104–109]. Mid2 organizes the septin ring that forms on either side of the septum barrier [106]. In the absence of the septin ring or mid2, the septum digesting glucanases are not localized properly to the base of the primary septum [94]. The localization of the septin ring and the exocyst complex at the division site requires Rho4 GTPase [100]. The septin and exocyst localizes the GEF Gef3 which in turn activates Rho4 [102, 105]. It is tempting to speculate that the septins may act as a landmark to guide Rho4 activation, to ensure that the fusion of vesicles containing the glucanases Agn1 and Eng1 occurs only at the base of the primary septum to promote cell separation and prevent cell lysis. Expression of Mid2 as well as the glucanases is regulated by the transcription factor Ace2 in a cell cycle dependent manner thus ensuring that cell separation occurs after septation [110, 111].
These different trafficking events occur in a temporally sequential manner at distinct regions of the division site. Membrane trafficking events during cytokinesis are thus tightly regulated spatiotemporally to coordinate different cytokinetic events.
Future Avenues
In fission yeast, the septum plays an important role in cytokinesis and proper septum formation and cell separation are necessary for cell integrity and viability. By acting as a landmark for septum synthesis, the ring ensures that the septum is always built adjacent to the actomyosin ring, thus maintaining the fidelity of the division site positioning. As described above, membrane trafficking events are critical for cytokinesis. Moreover, proper cytokinesis requires spatiotemporal regulation of different membrane trafficking events. Recent advances raise several intriguing questions in the field. Failure in any one of the cytokinetic steps can lead to cell death. Furthermore, cytokinetic events need to occur in the proper and sequential manner in order to maintain cell integrity and successful cell separation. How does the cell precisely organize the different events during cytokinesis? Evidence indicate that signaling pathways including the SIN pathway and the Cdc42/Rho GTPases regulate the different steps in cytokinesis [17, 45, 69]. How are these different signaling pathways regulated to ensure that each step during cytokinesis occurs in the correct order and in coordination with nuclear division? How are Cdc42 and Rho GTPases regulated to organize cytokinesis events? During cytokinesis, the septum building apparatus is deposited at the ring membrane interface and also to the ingressing membrane. Membrane deposition occurs at the ingressing membrane barrier and digestive enzymes are delivered to the rim of the membrane barrier. How do signaling pathways spatially restrict these processes to distinct sites of action to prevent ectopic cell wall deposition and cell lysis? Addressing these questions will provide a better understanding of the mechanistic details of cytokinesis and provide insights into how the cell regulates multiple membrane trafficking-mediated polarization events. Thus, knowledge gathered on the mechanistic details of membrane trafficking and polarization during cytokinesis will provide a paradigm for understanding fundamental principles of cell polarity in general.
The cell wall is analogous to extracellular matrix in higher eukaryotes. Interestingly, extracellular matrix remodeling has been shown to be required for cytokinesis in other systems. In C. elegans embryos, germ cells, and in pre-implantation mouse embryos, cytokinesis requires extracellular matrix remodeling [112–115]. In C. elegans, a defect in chondroitin biosynthesis results in the failure to initiate cytokinesis during early embryogenesis [114]. In another study it was shown that extracellular hemicentins localize to the cleavage furrow in the germline of C. elegans and also in mouse embryos [112]. In mutants lacking hemicentin, the cleavage furrow initiates but later regresses, leading to cytokinetic failure [112]. It is unclear how these extracellular matrix components contribute to cytokinesis. A more recent study has also shown that optogenetically activated RhoA at the medial region of C. elegans embryo initiates actomyosin ring constriction but fails to complete this process [116]. It is unclear why the cleavage furrow in these cells fail cytokinesis. One explanation could be that premature initiation of the cleavage furrow by RhoA activation fails because the extracellular matrix is not yet properly remodeled to facilitate cytokinesis. Thus, coupling of extracellular matrix remodeling and actomyosin ring mediated furrow formation may be a prevalent feature of cytokinesis in eukaryotes. Further investigations will reveal why and how extracellular matrix promotes cytokinesis in other organisms.
Acknowledgments
We thank the following funding agencies for support. MD is supported by National Science Foundation (1616495). U.N.O. was supported by National Institute of Health-IMSD (R25GM086761) and is currently supported by a National Science Foundation Graduate Research Fellowship (DGE-1452154).
Footnotes
Conflict of Interest
There authors declare that they have no conflict of interests.
References
- 1.Li R, Cytokinesis in development and disease: variations on a common theme. Cell Mol Life Sci, 2007. 64(23): p. 3044–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guertin DA, Trautmann S, and McCollum D, Cytokinesis in eukaryotes. Microbiol Mol Biol Rev, 2002. 66(2): p. 155–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pollard TD, Mechanics of cytokinesis in eukaryotes. Curr Opin Cell Biol, 2010. 22(1): p. 50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollard TD and Wu JQ, Understanding cytokinesis: lessons from fission yeast. Nat Rev Mol Cell Biol, 2010. 11(2): p. 149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coffman VC, et al. , Roles of formin nodes and myosin motor activity in Mid1p-dependent contractile-ring assembly during fission yeast cytokinesis. Mol Biol Cell, 2009. 20(24): p. 5195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laporte D, et al. , Assembly and architecture of precursor nodes during fission yeast cytokinesis. J Cell Biol, 2011. 192(6): p. 1005–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rincon SA, et al. , SIN-Dependent Dissociation of the SAD Kinase Cdr2 from the Cell Cortex Resets the Division Plane. Curr Biol, 2017. 27(4): p. 534–542. [DOI] [PubMed] [Google Scholar]
- 8.Vavylonis D, et al. , Assembly mechanism of the contractile ring for cytokinesis by fission yeast. Science, 2008. 319(5859): p. 97–100. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y, Yan H, and Balasubramanian MK, Assembly of normal actomyosin rings in the absence of Mid1p and cortical nodes in fission yeast. J Cell Biol, 2008. 183(6): p. 979–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu JQ, et al. , Assembly of the cytokinetic contractile ring from a broad band of nodes in fission yeast. J Cell Biol, 2006. 174(3): p. 391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.JC GC, et al. , Specific detection of fission yeast primary septum reveals septum and cleavage furrow ingression during early anaphase independent of mitosis completion. PLoS Genet, 2018. 14(5): p. e1007388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovar DR, et al. , The fission yeast cytokinesis formin Cdc12p is a barbed end actin filament capping protein gated by profilin. J Cell Biol, 2003. 161(5): p. 875–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yonetani A, et al. , Regulation and targeting of the fission yeast formin cdc12p in cytokinesis. Mol Biol Cell, 2008. 19(5): p. 2208–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamasaki T, Osumi M, and Mabuchi I, Three-dimensional arrangement of F-actin in the contractile ring of fission yeast. J Cell Biol, 2007. 178(5): p. 765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J, et al. , Nonmedially assembled F-actin cables incorporate into the actomyosin ring in fission yeast. J Cell Biol, 2012. 199(5): p. 831–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coffman VC, et al. , The formins Cdc12 and For3 cooperate during contractile ring assembly in cytokinesis. J Cell Biol, 2013. 203(1): p. 101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simanis V, Pombe’s thirteen - control of fission yeast cell division by the septation initiation network. J Cell Sci, 2015. 128(8): p. 1465–74. [DOI] [PubMed] [Google Scholar]
- 18.Bohnert KA, et al. , SIN-dependent phosphoinhibition of formin multimerization controls fission yeast cytokinesis. Genes Dev, 2013. 27(19): p. 2164–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts-Galbraith RH, et al. , Dephosphorylation of F-BAR protein Cdc15 modulates its conformation and stimulates its scaffolding activity at the cell division site. Mol Cell, 2010. 39(1): p. 86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CT, et al. , The SIN kinase Sid2 regulates cytoplasmic retention of the S. pombe Cdc14-like phosphatase Clp1. Curr Biol, 2008. 18(20): p. 1594–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clifford DM, et al. , The Clp1/Cdc14 phosphatase contributes to the robustness of cytokinesis by association with anillin-related Mid1. J Cell Biol, 2008. 181(1): p. 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu JQ, et al. , Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev Cell, 2003. 5(5): p. 723–34. [DOI] [PubMed] [Google Scholar]
- 23.Cortes JC, et al. , The novel fission yeast (1,3)beta-D-glucan synthase catalytic subunit Bgs4p is essential during both cytokinesis and polarized growth. J Cell Sci, 2005. 118(Pt 1): p. 157–74. [DOI] [PubMed] [Google Scholar]
- 24.Laporte D, Zhao R, and Wu JQ, Mechanisms of contractile-ring assembly in fission yeast and beyond. Semin Cell Dev Biol, 2010. 21(9): p. 892–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortes JC, et al. , Localization of the (1,3)beta-D-glucan synthase catalytic subunit homologue Bgs1p/Cps1p from fission yeast suggests that it is involved in septation, polarized growth, mating, spore wall formation and spore germination. J Cell Sci, 2002. 115(Pt 21): p. 4081–96. [DOI] [PubMed] [Google Scholar]
- 26.Cortes JC, et al. , The (1,3)beta-D-glucan synthase subunit Bgs1p is responsible for the fission yeast primary septum formation. Mol Microbiol, 2007. 65(1): p. 201–17. [DOI] [PubMed] [Google Scholar]
- 27.Munoz J, et al. , Extracellular cell wall beta(1,3)glucan is required to couple septation to actomyosin ring contraction. J Cell Biol, 2013. 203(2): p. 265–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cortes JC, et al. , Fission yeast septation. Commun Integr Biol, 2016. 9(4): p. e1189045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia Cortes JC, et al. , The Cell Biology of Fission Yeast Septation. Microbiol Mol Biol Rev, 2016. 80(3): p. 779–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dekker N, et al. , Role of the alpha-glucanase Agn1p in fission-yeast cell separation. Mol Biol Cell, 2004. 15(8): p. 3903–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin-Cuadrado AB, et al. , The endo-beta-1,3-glucanase eng1p is required for dissolution of the primary septum during cell separation in Schizosaccharomyces pombe. J Cell Sci, 2003. 116(Pt 9): p. 1689–98. [DOI] [PubMed] [Google Scholar]
- 32.Pollard TD, The value of mechanistic biophysical information for systems-level understanding of complex biological processes such as cytokinesis. Biophys J, 2014. 107(11): p. 2499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee IJ, Coffman VC, and Wu JQ, Contractile-ring assembly in fission yeast cytokinesis: Recent advances and new perspectives. Cytoskeleton (Hoboken), 2012. 69(10): p. 751–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald NA, et al. , Nanoscale architecture of the Schizosaccharomyces pombe contractile ring. Elife, 2017. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laplante C, et al. , Molecular organization of cytokinesis nodes and contractile rings by super-resolution fluorescence microscopy of live fission yeast. Proc Natl Acad Sci U S A, 2016. 113(40): p. E5876–E5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Humbel BM, et al. , In situ localization of beta-glucans in the cell wall of Schizosaccharomyces pombe. Yeast, 2001. 18(5): p. 433–44. [DOI] [PubMed] [Google Scholar]
- 37.Ribas JC, et al. , Isolation and characterization of Schizosaccharomyces pombe mutants defective in cell wall (1–3)beta-D-glucan. J Bacteriol, 1991. 173(11): p. 3456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cortes JC, et al. , Fission yeast Ags1 confers the essential septum strength needed for safe gradual cell abscission. J Cell Biol, 2012. 198(4): p. 637–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katayama S, et al. , Fission yeast alpha-glucan synthase Mok1 requires the actin cytoskeleton to localize the sites of growth and plays an essential role in cell morphogenesis downstream of protein kinase C function. J Cell Biol, 1999. 144(6): p. 1173–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hochstenbach F, et al. , Identification of a putative alpha-glucan synthase essential for cell wall construction and morphogenesis in fission yeast. Proc Natl Acad Sci U S A, 1998. 95(16): p. 9161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin V, et al. , Bgs3p, a putative 1,3-beta-glucan synthase subunit, is required for cell wall assembly in Schizosaccharomyces pombe. Eukaryot Cell, 2003. 2(1): p. 159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ren L, et al. , The Cdc15 and Imp2 SH3 domains cooperatively scaffold a network of proteins that redundantly ensure efficient cell division in fission yeast. Mol Biol Cell, 2015. 26(2): p. 256–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arasada R and Pollard TD, Contractile ring stability in S. pombe depends on F-BAR protein Cdc15p and Bgs1p transport from the Golgi complex. Cell Rep, 2014. 8(5): p. 1533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J, et al. , The localization of the integral membrane protein Cps1p to the cell division site is dependent on the actomyosin ring and the septation-inducing network in Schizosaccharomyces pombe. Mol Biol Cell, 2002. 13(3): p. 989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei B, et al. , Unique Spatiotemporal Activation Pattern of Cdc42 by Gef1 and Scd1 Promotes Different Events during Cytokinesis. Mol Biol Cell, 2016. 27(8): p. 1235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts-Galbraith RH, et al. , The SH3 domains of two PCH family members cooperate in assembly of the Schizosaccharomyces pombe contractile ring. J Cell Biol, 2009. 184(1): p. 113–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davidson R, Pontasch JA, and Wu JQ, Sbg1 Is a Novel Regulator for the Localization of the beta-Glucan Synthase Bgs1 in Fission Yeast. PLoS One, 2016. 11(11): p. e0167043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sethi K, et al. , A New Membrane Protein Sbg1 Links the Contractile Ring Apparatus and Septum Synthesis Machinery in Fission Yeast. PLoS Genet, 2016. 12(10): p. e1006383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martins IM, et al. , Differential activities of three families of specific beta(1,3)glucan synthase inhibitors in wild-type and resistant strains of fission yeast. J Biol Chem, 2011. 286(5): p. 3484–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castro C, et al. , Papulacandin B resistance in budding and fission yeasts: isolation and characterization of a gene involved in (1,3)beta-D-glucan synthesis in Saccharomyces cerevisiae. J Bacteriol, 1995. 177(20): p. 5732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cortes JC, et al. , Cooperation between Paxillin-like Protein Pxl1 and Glucan Synthase Bgs1 Is Essential for Actomyosin Ring Stability and Septum Formation in Fission Yeast. PLoS Genet, 2015. 11(7): p. e1005358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin-Garcia R, et al. , Paxillin-Mediated Recruitment of Calcineurin to the Contractile Ring Is Required for the Correct Progression of Cytokinesis in Fission Yeast. Cell Rep, 2018. 25(3): p. 772–783 e4. [DOI] [PubMed] [Google Scholar]
- 53.Le Goff X, Woollard A, and Simanis V, Analysis of the cps1 gene provides evidence for a septation checkpoint in Schizosaccharomyces pombe. Mol Gen Genet, 1999. 262(1): p. 163–72. [DOI] [PubMed] [Google Scholar]
- 54.Proctor SA, et al. , Contributions of turgor pressure, the contractile ring, and septum assembly to forces in cytokinesis in fission yeast. Curr Biol, 2012. 22(17): p. 1601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu J, et al. , Drc1p/Cps1p, a 1,3-beta-glucan synthase subunit, is essential for division septum assembly in Schizosaccharomyces pombe. Genetics, 1999. 153(3): p. 1193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stachowiak MR, et al. , Mechanism of cytokinetic contractile ring constriction in fission yeast. Dev Cell, 2014. 29(5): p. 547–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mishra M, et al. , In vitro contraction of cytokinetic ring depends on myosin II but not on actin dynamics. Nat Cell Biol, 2013. 15(7): p. 853–9. [DOI] [PubMed] [Google Scholar]
- 58.Cheffings TH, Burroughs NJ, and Balasubramanian MK, Actomyosin Ring Formation and Tension Generation in Eukaryotic Cytokinesis. Curr Biol, 2016. 26(15): p. R719–R737. [DOI] [PubMed] [Google Scholar]
- 59.Zhou Z, et al. , The contractile ring coordinates curvature-dependent septum assembly during fission yeast cytokinesis. Mol Biol Cell, 2015. 26(1): p. 78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thiyagarajan S, et al. , The fission yeast cytokinetic contractile ring regulates septum shape and closure. J Cell Sci, 2015. 128(19): p. 3672–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laplante C, et al. , Three Myosins Contribute Uniquely to the Assembly and Constriction of the Fission Yeast Cytokinetic Contractile Ring. Curr Biol, 2015. 25(15): p. 1955–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kitayama C, Sugimoto A, and Yamamoto M, Type II myosin heavy chain encoded by the myo2 gene composes the contractile ring during cytokinesis in Schizosaccharomyces pombe. J Cell Biol, 1997. 137(6): p. 1309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.May KM, et al. , Type II myosin involved in cytokinesis in the fission yeast, Schizosaccharomyces pombe. Cell Motil Cytoskeleton, 1997. 38(4): p. 385–96. [DOI] [PubMed] [Google Scholar]
- 64.Chang F, Woollard A, and Nurse P, Isolation and characterization of fission yeast mutants defective in the assembly and placement of the contractile actin ring. Journal of cell science, 1996. 109 (Pt 1): p. 131–42. [DOI] [PubMed] [Google Scholar]
- 65.Balasubramanian MK, et al. , Isolation and characterization of new fission yeast cytokinesis mutants. Genetics, 1998. 149(3): p. 1265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sohrmann M, et al. , The dmf1/mid1 gene is essential for correct positioning of the division septum in fission yeast. Genes Dev, 1996. 10(21): p. 2707–19. [DOI] [PubMed] [Google Scholar]
- 67.Bahler J, et al. , Role of polo kinase and Mid1p in determining the site of cell division in fission yeast. J Cell Biol, 1998. 143(6): p. 1603–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paoletti A and Chang F, Analysis of mid1p, a protein required for placement of the cell division site, reveals a link between the nucleus and the cell surface in fission yeast. Mol Biol Cell, 2000. 11(8): p. 2757–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roberts-Galbraith RH and Gould KL, Stepping into the ring: the SIN takes on contractile ring assembly. Genes Dev, 2008. 22(22): p. 3082–8. [DOI] [PubMed] [Google Scholar]
- 70.Hergovich A and Hemmings BA, Mammalian NDR/LATS protein kinases in hippo tumor suppressor signaling. Biofactors, 2009. 35(4): p. 338–45. [DOI] [PubMed] [Google Scholar]
- 71.McDonald NA, et al. , Oligomerization but Not Membrane Bending Underlies the Function of Certain F-BAR Proteins in Cell Motility and Cytokinesis. Dev Cell, 2015. 35(6): p. 725–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wachtler V, et al. , Cell cycle-dependent roles for the FCH-domain protein Cdc15p in formation of the actomyosin ring in Schizosaccharomyces pombe. Mol Biol Cell, 2006. 17(7): p. 3254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hachet O and Simanis V, Mid1p/anillin and the septation initiation network orchestrate contractile ring assembly for cytokinesis. Genes Dev, 2008. 22(22): p. 3205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu JQ and Pollard TD, Counting cytokinesis proteins globally and locally in fission yeast. Science, 2005. 310(5746): p. 310–4. [DOI] [PubMed] [Google Scholar]
- 75.Arellano M, Duran A, and Perez P, Rho 1 GTPase activates the (1–3)beta-D-glucan synthase and is involved in Schizosaccharomyces pombe morphogenesis. The EMBO journal, 1996. 15(17): p. 4584–91. [PMC free article] [PubMed] [Google Scholar]
- 76.Mutoh T, Nakano K, and Mabuchi I, Rho1-GEFs Rgf1 and Rgf2 are involved in formation of cell wall and septum, while Rgf3 is involved in cytokinesis in fission yeast. Genes Cells, 2005. 10(12): p. 1189–202. [DOI] [PubMed] [Google Scholar]
- 77.Morrell-Falvey JL, et al. , Cell wall remodeling at the fission yeast cell division site requires the Rho-GEF Rgf3p. J Cell Sci, 2005. 118(Pt 23): p. 5563–73. [DOI] [PubMed] [Google Scholar]
- 78.Tajadura V, et al. , Schizosaccharomyces pombe Rgf3p is a specific Rho1 GEF that regulates cell wall beta-glucan biosynthesis through the GTPase Rho1p. J Cell Sci, 2004. 117(Pt 25): p. 6163–74. [DOI] [PubMed] [Google Scholar]
- 79.Garcia P, et al. , Role of Rho GTPases and Rho-GEFs in the regulation of cell shape and integrity in fission yeast. Yeast, 2006. 23(13): p. 1031–43. [DOI] [PubMed] [Google Scholar]
- 80.Perez P, et al. , Fission yeast cell wall biosynthesis and cell integrity signalling. The Cell Surface, 2018. 4: p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Onwubiko UN, et al. , A Cdc42 GEF, Gef1, through endocytosis organizes F-BAR Cdc15 along the actomyosin ring and promotes concentric furrowing. J Cell Sci, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Estravis M, et al. , Cdc42 regulates multiple membrane traffic events in fission yeast. Traffic, 2011. 12(12): p. 1744–58. [DOI] [PubMed] [Google Scholar]
- 83.Hirota K, et al. , Gef1p and Scd1p, the Two GDP-GTP exchange factors for Cdc42p, form a ring structure that shrinks during cytokinesis in Schizosaccharomyces pombe. Mol Biol Cell, 2003. 14(9): p. 3617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martin-Garcia R, Coll PM, and Perez P, F-BAR domain protein Rga7 collaborates with Cdc15 and Imp2 to ensure proper cytokinesis in fission yeast. J Cell Sci, 2014. 127(Pt 19): p. 4146–58. [DOI] [PubMed] [Google Scholar]
- 85.Soto T, et al. , Rga4 modulates the activity of the fission yeast cell integrity MAPK pathway by acting as a Rho2 GTPase-activating protein. J Biol Chem, 2010. 285(15): p. 11516–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu Y, et al. , The F-BAR Domain of Rga7 Relies on a Cooperative Mechanism of Membrane Binding with a Partner Protein during Fission Yeast Cytokinesis. Cell Rep, 2019. 26(10): p. 2540–2548 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arasada R and Pollard TD, A role for F-BAR protein Rga7p during cytokinesis in S. pombe. J Cell Sci, 2015. 128(13): p. 2259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang N, et al. , Roles of the TRAPP-II Complex and the Exocyst in Membrane Deposition during Fission Yeast Cytokinesis. PLoS Biol, 2016. 14(4): p. e1002437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mulvihill DP, Edwards SR, and Hyams JS, A critical role for the type V myosin, Myo52, in septum deposition and cell fission during cytokinesis in Schizosaccharomyces pombe. Cell Motil Cytoskeleton, 2006. 63(3): p. 149–61. [DOI] [PubMed] [Google Scholar]
- 90.Martin SG, et al. , Regulation of the formin for3p by cdc42p and bud6p. Molecular biology of the cell, 2007. 18(10): p. 4155–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Estravis M, Rincon S, and Perez P, Cdc42 regulation of polarized traffic in fission yeast. Commun Integr Biol, 2012. 5(4): p. 370–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arasada R and Pollard TD, Distinct roles for F-BAR proteins Cdc15p and Bzz1p in actin polymerization at sites of endocytosis in fission yeast. Curr Biol, 2011. 21(17): p. 1450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang H, et al. , The multiprotein exocyst complex is essential for cell separation in Schizosaccharomyces pombe. Mol Biol Cell, 2002. 13(2): p. 515–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martin-Cuadrado AB, et al. , Role of septins and the exocyst complex in the function of hydrolytic enzymes responsible for fission yeast cell separation. Mol Biol Cell, 2005. 16(10): p. 4867–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garcia I, et al. , The alpha-glucanase Agn1p is required for cell separation in Schizosaccharomyces pombe. Biol Cell, 2005. 97(7): p. 569–76. [DOI] [PubMed] [Google Scholar]
- 96.Sipiczki M, et al. , Phylogenetic and comparative functional analysis of the cell-separation alpha-glucanase Agn1p in Schizosaccharomyces. Microbiology, 2014. 160(Pt 6): p. 1063–74. [DOI] [PubMed] [Google Scholar]
- 97.Martin-Cuadrado AB, et al. , The Schizosaccharomyces pombe endo-1,3-beta-glucanase Eng1 contains a novel carbohydrate binding module required for septum localization. Mol Microbiol, 2008. 69(1): p. 188–200. [DOI] [PubMed] [Google Scholar]
- 98.Roncero C and Sanchez Y, Cell separation and the maintenance of cell integrity during cytokinesis in yeast: the assembly of a septum. Yeast, 2010. 27(8): p. 521–30. [DOI] [PubMed] [Google Scholar]
- 99.Wang H, Tang X, and Balasubramanian MK, Rho3p regulates cell separation by modulating exocyst function in Schizosaccharomyces pombe. Genetics, 2003. 164(4): p. 1323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Perez P, Portales E, and Santos B, Rho4 interaction with exocyst and septins regulates cell separation in fission yeast. Microbiology, 2015. 161(Pt 5): p. 948–59. [DOI] [PubMed] [Google Scholar]
- 101.Bendezu FO and Martin SG, Actin cables and the exocyst form two independent morphogenesis pathways in the fission yeast. Mol Biol Cell, 2011. 22(1): p. 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang N, et al. , The Rho-GEF Gef3 interacts with the septin complex and activates the GTPase Rho4 during fission yeast cytokinesis. Mol Biol Cell, 2015. 26(2): p. 238–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Santos B, et al. , Rho4 GTPase is involved in secretion of glucanases during fission yeast cytokinesis. Eukaryot Cell, 2005. 4(10): p. 1639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.An H, et al. , Requirements of fission yeast septins for complex formation, localization, and function. Mol Biol Cell, 2004. 15(12): p. 5551–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Munoz S, Manjon E, and Sanchez Y, The putative exchange factor Gef3p interacts with Rho3p GTPase and the septin ring during cytokinesis in fission yeast. J Biol Chem, 2014. 289(32): p. 21995–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tasto JJ, Morrell JL, and Gould KL, An anillin homologue, Mid2p, acts during fission yeast cytokinesis to organize the septin ring and promote cell separation. J Cell Biol, 2003. 160(7): p. 1093–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sipiczki M, Splitting of the fission yeast septum. FEMS Yeast Res, 2007. 7(6): p. 761–70. [DOI] [PubMed] [Google Scholar]
- 108.Wu JQ, et al. , Cooperation between the septins and the actomyosin ring and role of a cell-integrity pathway during cell division in fission yeast. Genetics, 2010. 186(3): p. 897–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zheng S, et al. , Septins regulate the equatorial dynamics of the separation initiation network kinase Sid2p and glucan synthases to ensure proper cytokinesis. FEBS J, 2018. 285(13): p. 2468–2480. [DOI] [PubMed] [Google Scholar]
- 110.Alonso-Nunez ML, et al. , Ace2p controls the expression of genes required for cell separation in Schizosaccharomyces pombe. Mol Biol Cell, 2005. 16(4): p. 2003–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Petit CS, et al. , Ace2p contributes to fission yeast septin ring assembly by regulating mid2+ expression. J Cell Sci, 2005. 118(Pt 24): p. 5731–42. [DOI] [PubMed] [Google Scholar]
- 112.Xu X and Vogel BE, A secreted protein promotes cleavage furrow maturation during cytokinesis. Curr Biol, 2011. 21(2): p. 114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hwang HY, et al. , Caenorhabditis elegans early embryogenesis and vulval morphogenesis require chondroitin biosynthesis. Nature, 2003. 423(6938): p. 439–43. [DOI] [PubMed] [Google Scholar]
- 114.Olson SK, et al. , Identification of novel chondroitin proteoglycans in Caenorhabditis elegans: embryonic cell division depends on CPG-1 and CPG-2. J Cell Biol, 2006. 173(6): p. 985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jordan SN, Olson S, and Canman JC, Cytokinesis: thinking outside the cell. Curr Biol, 2011. 21(3): p. R119–21. [DOI] [PubMed] [Google Scholar]
- 116.Wagner E and Glotzer M, Local RhoA activation induces cytokinetic furrows independent of spindle position and cell cycle stage. J Cell Biol, 2016. 213(6): p. 641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Villalobos-Duno H, et al. , Biochemical characterization of Paracoccidioides brasiliensis alpha-1,3-glucanase Agn1p, and its functionality by heterologous Expression in Schizosaccharomyces pombe. PLoS One, 2013. 8(6): p. e66853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nakano K, et al. , The small GTPase Rho4 is involved in controlling cell morphology and septation in fission yeast. Genes Cells, 2003. 8(4): p. 357–70. [DOI] [PubMed] [Google Scholar]