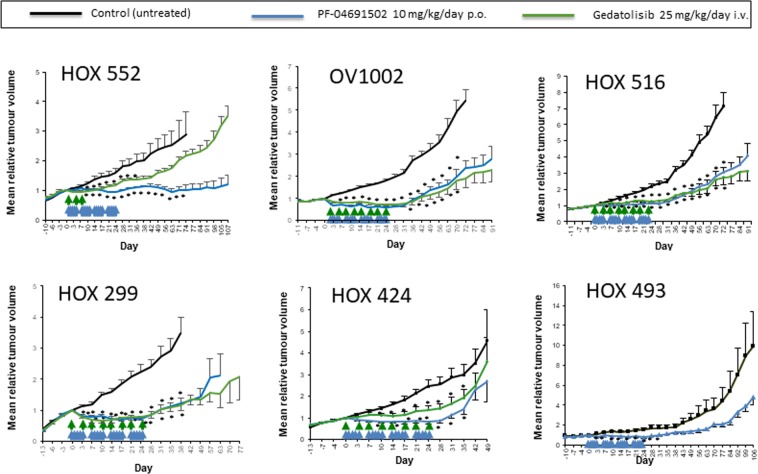
Figure 1.
Antitumour activity of PF-04691502 and gedatolisib against a panel of human ovarian cancer xenografts. Both drugs were evaluated against the HOX 552, OV1002, HOX 516, HOX 299, HOX 424 models. PF-04691502 only was tested against the HOX 493 model. PF-04691502 (10 mg/kg/day p.o.) and gedatolisib (25 mg/kg/day i.v.) were administered on the days indicated by arrows. PF-04691502 was administered over 4 weeks (days 0–4; 7–11; 14–18; 21–25) against all 6 models while gedatolisib (25 mg/kg) was given on days 0, 4 and 8 only to HOX 552 but then extended to days 0, 4, 7, 11, 14, 18, 21 and 25 for OV1002, HOX516, HOX299, HOX424. Mean (+/− S.E.) tumour volumes are shown. For OV1002 and HOX 299, all treatment points shown were statistically different from control (ANOVA followed by Tukey post-test; *p < 0.05). For HOX 516, all treatment points beyond Day 3 were statistically different from control. For HOX 552, all treatment points were statistically different from control beyond Day 10 until Day 36 for gedatolisib and Day 71 for PF-04691502. For HOX424, all treatment points after Day 7 and before Day 35 were statistically different from control. For HOX 493, all treatment points were statistically different from control until Day 31.