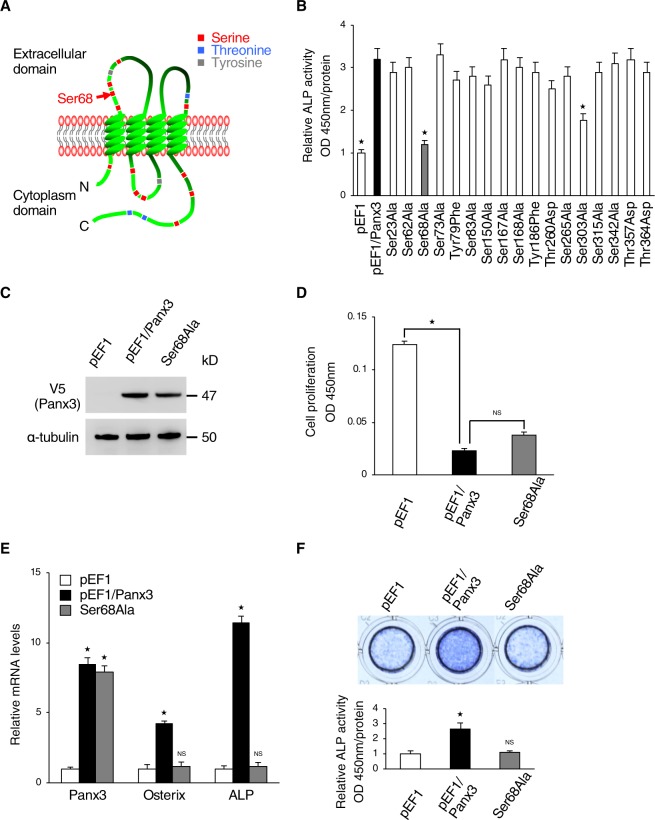
Figure 2.
Ser68 is candidate phosphorylation residue for regulation of Panx3 function in osteoblast differentiation. (A) The locations of 17 candidate phosphorylation residues in the Panx3 protein. Ser68 (red arrow) is located in the first extracellular loop. (B) Screening of candidate sites of interest by ALP activity assays with mutation constructs in each of the 17 candidate sites. C2C12 cells transiently transfected with each mutation construct were cultured with DMEM + BMP2 (300 ng/ml) for 2 days. Normalized by each Panx3 protein expression detected by western blotting. (C) Establishment of Ser68Ala stably transfected C2C12 cells. Western blot of Panx3 expression in Ser68Ala cells compared with normal Panx3 stably transfected C2C12 cells (pEF1/Panx3). Western blot was perfomed by at least three independent experiments. Full blot is shown in the Supplemental Information (Full Original Blots-II). (D) Proliferation of pEF1/Panx3 or Ser68Ala stably transfected cells. Cells were cultured in DMEM media for 2 days. (E) C2C12 cells stably transfected with a control pEF1 vector, pEF1/Panx3 or Ser68Ala were cultured with BMP2 (300 ng/ml) for 3 days. Total RNA then was extracted and mRNA levels of Panx3 and the osteoblast differentiation marker, Osterix, and ALP were analyzed by quantitative RT-PCR. (F) Ser68Ala inhibited Panx3-promoted ALP activity. Representative ALP staining (top) and its quantitative data (bottom). Cells stably transfected with pEF1/Panx3 and Ser68 were cultured with BMP2 (300 ng/ml) for 3 days. *P < 0.01. NS, nonsignificant. Error bars represent the mean ± SD; n = 3.