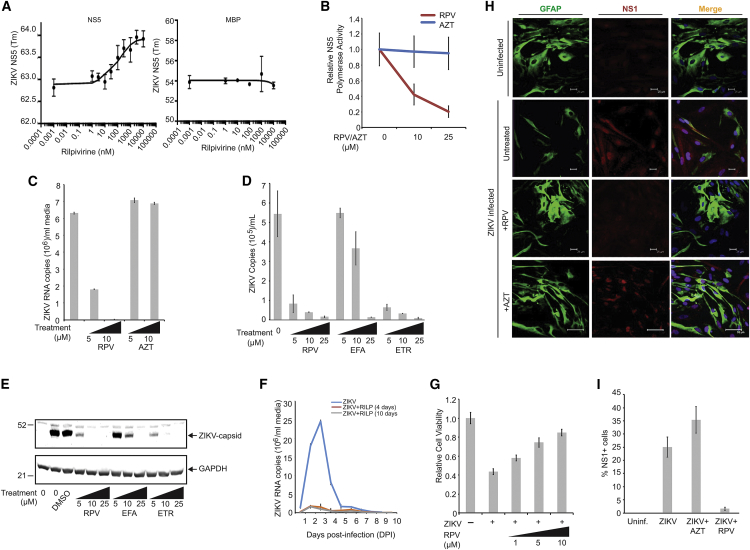
Figure 3.
Inhibition of ZIKV Infection of Human Primary Culture of Brain Cells by RPV
(A) Interaction of ZIKV NS5 protein with RPV using protein thermal shift assays. The relative fluorescence emitted by the thermal shift dye is recorded during the temperature ramp phase and plotted versus temperature (left panel). Tm value was calculated from the derivative of the thermal melt curves and plotted as effects of various concentrations of RPV, as described in the Materials and Methods. Determination of binding interaction of maltose binding protein (MBP) moiety of NS5-MBP with RPV using differential scanning fluorimetry (DSF) showed no association of MBP with RPV (right panel). (B) RPV inhibits RNA polymerase activity of ZIKV NS5 protein. 1 μg ZIKV RNA and 500 ng recombinant NS5 protein were added to the reaction buffer, in the presence and absence of RPV or AZT at 10- or 25-ng concentration, and analyzed as described in the Materials and Methods. Relative NS5 activity was calculated from three independent experiments and is shown as a trend-line graph. (C) PHFA cells were infected with ZIKV (0.5 plaque-forming unit [PFU]). Cells were treated daily with increasing concentrations of RPV and AZT (5 and 10 μg/mL). At 3 dpi, ZIKV RNA copies in the growth media were analyzed by real time qRT-PCR and are shown as a bar graph. (D) PHFA cells were infected with ZIKV (0.1 PFU). Cells were treated daily with increasing concentrations of RPV, EFA, and ETR (5, 10, and 25 μg/mL). At 3 dpi, ZIKV RNA copies in the growth media were analyzed by real time qRT-PCR and are shown as a bar graph. (E) Whole-cell protein extracts were also prepared from the same set of experiments from (D) and analyzed by western blotting for the detection of ZIKV capsid protein. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was probed in the same membranes and is shown as a loading control. (F) PHFA cells were divided into three groups. The first group was infected with ZIKV and mock treated at 24-h intervals for 10 days. The second group of cells was infected with ZIKV (0.1 PFU) and treated with RPV (5 μg/mL) at 24-h intervals for only 4 days. The third group was also infected with ZIKV (0.1 PFU) and treated with RPV (5 μg/mL) at 24-h intervals for 10 days. The growth media of cells were collected every day, starting from 1 dpi up to 10 dpi, and analyzed by real time qRT-PCR. ZIKV viral loads were determined as ZIKV RNA copies per milliliter culture media and are shown as a trend-line graph for each group. (G) PHFAs were infected with ZIKV (0.1 PFU) and treated with RPV at 1-, 5-, and 10-μg/mL concentrations for 4 days. PHFA cell viability was analyzed by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay and is shown as a bar graph from three independent experiments. (H) PHFA cells were infected with ZIKV (0.1 PFU) and treated daily with RPV and AZT. At 4 dpi, cells were fixed and processed by immunocytochemistry for the detection of cellular GFAP (green) and viral NS1 (red) proteins, as described in the Materials and Methods. (I) DAPI-positive and NS1-immunoreactive cells from studies shown in (H) were counted in at least three different stainings of the conditions using Photoshop, and percentages of NS1 cells were graphed.