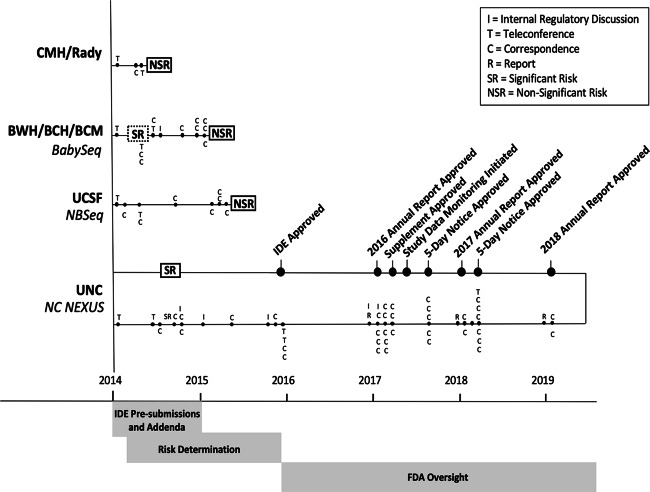
Fig. 1. Comparative timelines of FDA interactions and decision-making with the 3 sites (Rady, BWH/BCH/BCM and UCSF) that were determined to be Non-Significant Risk (NSR) and with the remaining site (UNC) in which Significant Risk (SR) was determined.
The phases of FDA–NSIGHT interaction were: IDE Pre-submission, in which all sites participated and submission of Pre-sub addenda, if needed by FDA; Risk determination; and ongoing FDA oversight for the NC NEXUS study (UNC). FDA-initiated activities are shown below the axis for each site, where as NSIGHT site-initiated activities are indicated above the axis for each site.