Fig. 1.
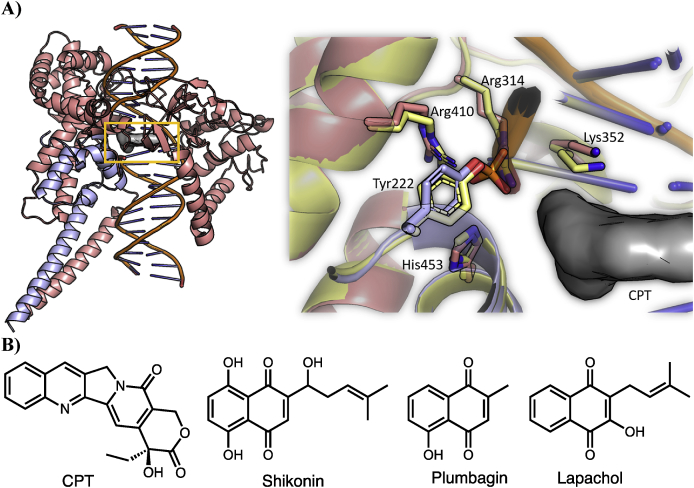
A) Structure of LTopIB interaction complexes. LTopIB, unlike human's, is atypically composed of two different subunits, which have to be assembled in the pathogen to reconstitute the enzyme activity. One of the subunits contains the four amino acids of the active site and the other contains the catalytic amino acid (Tyr222), which acts by breaking one DNA strand into a specific nucleotide sequence (Villa et al., 2003). Left: Structure of the Leishmania TopIB-DNA-CPT ternary complex. The model was built based on PDB: 1T8I structure (See methods for further details). The two subunits are displayed with different colors (salmon and blue) and CPT, which is intercalated between base pairs of DNA, is shown as a grey surface. The active site of LTopIB is located inside the yellow rectangle. Right: Structure of the active site of LTop1B. The important catalytic residues are displayed atomistically, and CPT is displayed as a grey surface. The color scheme is the same used for the left panel. To show the structural similarity between the active site of hTopIB and LTopIB, the structure of the human isoform is shown in yellow (PDB 1TL8). Tyr222 is phosphorylated in our model, the same as in the hTopIB structure. B) Structures of several inhibitors of topoisomerase IB. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)