Abstract
The aim of the present study was to evaluate the oncologic safety and reproductive outcome in patients with stage I epithelial ovarian cancer (EOC) treated with fertility-sparing surgery (FSS). Women aged ≤40 years with stage I EOC who had undergone FSS between 2000 and 2010 were retrospectively reviewed. Survival was examined using the Kaplan-Meier method and statistical significance was analyzed using the log-rank test. A total of 29 EOC patients (stage IA, n=14; stage IC1 n=6; stage IC3, n=9) from seven participating institutions belonging to the Tohoku Gynecologic Cancer Unit were enrolled. After a median follow-up duration of 60.6 months (range, 6–135 months), five patients (17.2%) experienced tumor recurrence. The respective five-year relapse-free survival (RFS) and overall survival (OS) rates were 90.9 and 100% for stage IA/IC1, and 43.8 and 87.5% for stage IC3. Significant differences in RFS were observed between stage IA/IC1 and IC3 patients (P=0.026). However, there was no significant difference in OS between patients with 1A/1C1 and those with 1C3 (P=0.712). After FSS, seven pregnancies occurred in five patients, which resulted in the birth of six healthy children. The results of the present study confirmed that FSS may be an acceptable treatment method for stage IA and IC1 EOC, exhibiting a favorable reproductive outcome. However, the safety of FSS for treating stage IC3 EOC is uncertain and warrants further investigation.
Keywords: epithelial ovarian cancer, stage I, fertility sparing surgery, oncologic safety, reproductive outcome
Introduction
Epithelial ovarian cancer (EOC) can occur at any age but is most commonly diagnosed in postmenopausal females, as the peak incidence is between the ages of 50 and 60 years. Standard EOC management involves primary surgery, including total abdominal hysterectomy, bilateral salpingo-oophorectomy, pelvic/para-aortic lymph node (LN) dissection, omentectomy and tumor debulking, followed by taxane/platinum-based adjuvant chemotherapy. Recently, women tend to give birth to their first child at an older age, and the diagnosis of EOC before childbearing has become more frequent. Because approximately 14% of all EOC cases occur in women aged under 40 years, some patients may choose fertility preservation (1). Fertility-sparing surgery (FSS), with preservation of the contralateral ovary and uterus, for women of reproductive age has been indicated for patients with stage I EOC (2). It is estimated that 7–8% of all stage I EOC patients are younger than 35 years of age (3).
According to the recent National Comprehensive Cancer Network (NCCN) guidelines, patients with unilateral stage (IA or IC) EOC based on comprehensive surgical staging can undergo FSS, regardless of grade (G) and histology (4). The present clinical guidelines of the European Society of Medical Oncology (ESMO) state that young women with stage IA or IC and favorable histological characteristics, such as non-clear cell carcinoma (CCC) and G1 or G2 tumors, are subject to FSS only following complete surgical staging that includes lymphadenectomy (5). However, regarding patients with high risk prognostic factors such as G3/CCC or stage IC subtypes, the safety of FSS is controversial. In the present study, we investigated the recurrence rates, survival, reproductive and obstetrical outcomes for women with stage I EOC treated with FSS.
Patients and methods
Study design
After obtaining the institutional review board approval of each of the seven participating institutions belonging to the Tohoku Gynecologic Cancer Unit (TGCU), the medical records of 29 patients with stage I EOC who had undergone FSS between 2000 and 2010 were retrospectively analyzed.
Participants
Additional criteria included that all patients were aged ≤40 years at the time of initial diagnosis, strongly desired to retain their fertility, and were informed about the possible risks and benefits of FSS. Exclusion criteria were non-epithelial histological type tumors (germ cell tumors and sex-cord stromal tumors), borderline malignant tumor, unclassified adenocarcinoma, and advanced EOC (≥stage II). All patients underwent FSS, such as unilateral oophorectomy or ovarian cystectomy with conservation of the uterus and contralateral ovary. Pathological diagnosis for histologic cell type, tumor differentiation and disease staging were performed in each institution. Staging was determined according to the International Federation of Gynecology and Obstetrics (FIGO) classification (2014). Centralized pathological review by a pathologist was performed at Fukushima Medical University Hospital to confirm tumor histology type and histologic differentiation. Following completion of primary treatment, patients were examined every 1–3 months during the first 2 years, every 3–6 months during the next 3 years and yearly thereafter. Recurrences were diagnosed during regular follow-up visits and confirmed on computed tomographic and/or magnetic resonance imaging scans.
All patients had provided written informed consent for surgery. The present study was approved by the Research Ethics Committee of each of the seven participating institutions belonging to the TGCU: Fukushima Medical University School of Medicine (Fukushima, Japan), Tohoku University Graduate School of Medicine (Sendai, Japan), Miyagi Cancer Center (Natori, Japan), Yamagata University School of Medicine (Yamagata, Japan), Iwate Medical University School of Medicine (Morioka, Japan), Akita University School of Medicine (Akita, Japan) and Hirosaki University School of Medicine (Hirosaki, Japan).
Statistical analysis
Overall survival (OS) and relapse-free survival (RFS) were evaluated as clinical outcomes. Survival distributions were calculated using the Kaplan-Meier method, and statistical significance was determined using the log-rank test. Values of P<0.05 were considered statistically significant. Statistical analysis of data was performed using SPSS 25 (IBM Corp., Armonk, NY, USA).
Results
Clinicopathological characteristics
A total of 29 patients with stage I EOC who were treated with FSS were enrolled in the present study. The clinicopathological characteristics of all patients are shown in Table I. The mean age was 27.2 years (range, 12 to 39 years), and the numbers of patients with stage IA and stage IC EOC were 14 and 15, respectively. The most frequent histological type was mucinous (16 cases, 55.2%), followed by serous (five, 17.2%) and endometrioid (five, 17.2%). Tumor differentiation was 13 (44.8%), 10 (34.5%) and 6 (20.7%) for G1, G2 and G3/CCC, respectively. Adjuvant chemotherapy was given to 19 (65.5%) patients with stage IA (7/14, 50%) and stage IC (12/15, 80%) EOC (data not shown). Eighteen patients underwent platinum-based chemotherapy, and one patient underwent oral etoposide. After a median follow-up of 60.6 months (range, 6–135 months), the clinical outcomes were as follows: 26 patients (89.7%) had no evidence of disease (NED); one (3.4%) patient was alive with disease (AWD); and two patients (6.9%) were dead of disease (DOD). Twenty-one patients did not experience a change in menstruation status after treatment, and two patients had amenorrhea. After FSS, five patients achieved seven pregnancies, resulting in the birth of six healthy children. Among the five patients, four had received platinum-based chemotherapy, and their babies showed no congenital malformations. As for primary symptoms, abdominal symptoms accounted for over 80% of all symptoms followed by atypical genital bleeding (10.3%).
Table I.
Clinicopathlogical characterstics of patients treated with FSS.
| Mean age, years (range) | 27.2 (12–39) |
| Surgical procedures, n (%) | |
| Cystectomy | 1 (3.5) |
| USO | 10 (34.5) |
| USO, OMT | 3 (10.3) |
| USO, OMT, LA | 15 (51.7) |
| Tumor histology, n (%) | |
| Mucinous | 16 (55.2) |
| Serous | 5 (17.2) |
| Endometrioid | 5 (17.2) |
| Clear cell | 3 (10.4) |
| Grade, an | |
| G1 | 13 |
| G2 | 10 |
| G3 | 3 |
| FIGO stage, n (%) | |
| IA | 14 (48.3) |
| G1 | 6 |
| G2 | 5 |
| G3 | 2 |
| Clear cell | 1 |
| IC1 | 6 (20.7) |
| G1 | 3 |
| G2 | 3 |
| G3 | 0 |
| Clear cell | 0 |
| IC3 | 9 (31.0) |
| G1 | 4 |
| G2 | 2 |
| G3 | 1 |
| Clear cell | 2 |
| Adjuvant chemotherapy, n (%) | |
| Yes | 19 (65.5) |
| No | 10 (34.5) |
| Recurrence, n (%) | |
| No | 24 (82.3) |
| Yes | 5 (17.2) |
| Clinical outcome, n (%) | |
| NED | 26 (89.7) |
| AWD | 1 (3.4) |
| DOD | 2 (6.9) |
| Menstruation, n | |
| Regular | 21 |
| Irregular | 1 |
| Amenorrhea | 2 |
| Unknown | 5 |
| Reproductive outcome, bn | |
| Parity | 7 |
| Primary symptom, cn (%) | |
| Birth | 6 |
| Abdominal symptom | 24 (82.8) |
| Atypical genital bleeding | 3 (10.3) |
| Medical examination | 2 (6.9) |
| Unknown | 1 (3.4) |
Clear cell is not graded.
Pregnancy in 5 patients, Birth in 4 patients.
One patient exhibited two symptoms. USO, unilateral salpingo-oophrectomy; OMT, omentectomy; LA, lymphadenectomy; NED, no evidence of disease; AWD, alive with disease; DOD, dead of disease.
Clinical outcome of FSS in patients with stage I EOC
Recurrence was identified during the follow-up period in five patients (17.2%) whose clinical details are described in Table II. The recurrence sites were: the pelvis n=2; pelvic LN n=1; cervical LN n=1; and pelvic LN and peritoneum n=1. Median duration from primary treatment to recurrence was 43.8 months (range, 17–55 months). After all five patients with recurrences were treated as outlined in Table II, two patients had NED, one patient was AWD, and two patients were DOD at 23 and 71 months after diagnosis. The estimated survival rate of all patients was 95.7% at five years and 89.3% at ten years.
Table II.
Clinical details of relapsed patients.
| Case | Age | Stage | Histology | Grade | Adjuvant chemotherapy | Site of recurrence | RFS (mo) | Salvage recurrence | Status | OS (mo) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 33 | IC3 | Serous | 1 | Yes | Pelvis | 40 | Radical surgery | NED | 119 |
| 2 | 36 | IC3 | Clear cell | Yes | Pelvic LN | 55 | Radical surgery | NED | 107 | |
| 3 | 27 | IC3 | Serous | 3 | Yes | Pelvis | 54 | Radical surgery | AWD | 104 |
| 4 | 19 | IC3 | Mucinous | 2 | No | Cervical LN | 17 | CCRT | DOD | 23 |
| 5 | 21 | IA | Serous | 1 | Yes | Pelvic LN Peritoneum | 53 | Chemotherapy | DOD | 71 |
LN, lymph node; CCRT, concurrent chemoradiotherapy; NED, no evidence of disease; AWD, alive with disease; DOD, dead of disease; RFS, relapse-free survival; OS, overall survival.
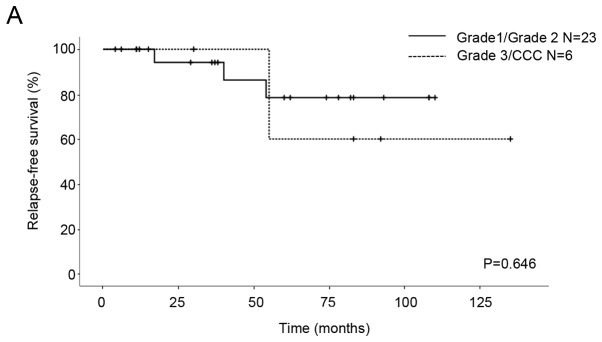
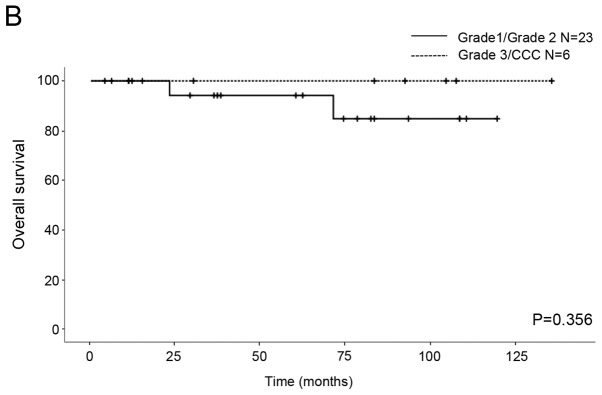
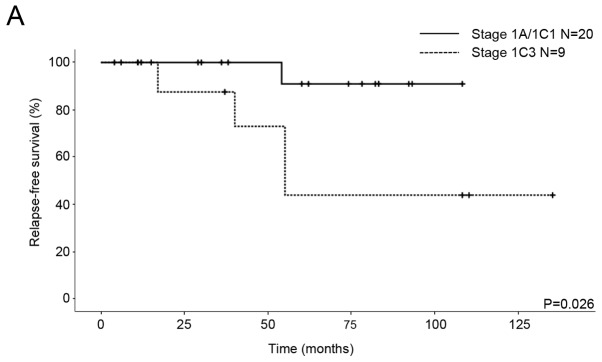
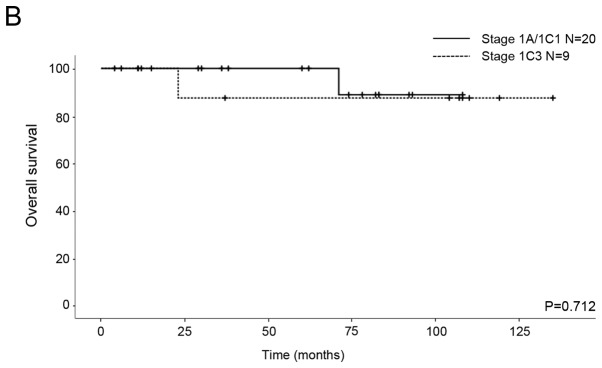
We compared the OS and RFS for histological differentiation and FIGO stage. All patients were classified into two subgroups from the point of view of prognostic factors. The first subgroup was G1/G2 vs. G3/CCC for histological differentiation and the second subgroup was IA/IC1 vs. 1C3 for stage. As for the patients with G1/G2 and G3/CCC, the five-year RFS rates were 78.4 and 60%, respectively, and the five-year OS rates were 94.1 and 100%, respectively. No significant differences in RFS and OS were seen between the patients (RFS P=0.646, OS P=0.356; Fig. 1). With regard to the patients with stage IA/IC1 and 1C3 EOC, the five-year RFS rates were 90.9 and 43.8%, respectively, and the five-year OS rates were 100 and 87.5%, respectively. Significant differences in RFS were seen between the patients (P=0.026; Fig. 2A), although there was no significant difference in OS (P=0.712; Fig. 2B).
Figure 1.
Kaplan-Meier survival curves of 29 patients with stage I EOC. (A) Relapse-free survival and (B) overall survival curves for grade 1/2 and grade 3/CCC. CCC, non-clear cell carcinoma.
Figure 2.
Kaplan-Meier survival curves of 29 patients with stage I EOC. (A) Relapse-free survival and (B) overall survival curves for stage IA/IC1 and stage IC3.
Discussion
The final goal of FSS is curable, safe and good reproductive and obstetrical outcomes in cases of early EOC in young women who desire childbearing. In our series, after a median follow-up of 60.6 months, the recurrence rate among the 29 stage I EOC patients who underwent FSS was 13% (5 of 29), which is similar to the majority of trials focusing on early-stage EOC, treated with either a radical approach or FSS, falling between the reported 4 and 15% (6). Schlaerth et al (7), reported that the five-year survival data for 123 patients who underwent surgical treatment for stage I EOC for fertility-preservation revealed no significant differences in five-year RFS (84% vs. 78%) or OS (84% vs. 82%) when compared to standard surgery. In addition, a recent study reported that FSS was not associated with increased risk of death in young women with stage I EOC compared with conventional surgery (8). The present study revealed a five-year RFS rate of 73.1% (data not shown) and an OS rate of 95.7%, which are consistent with the results of previous reports (7,9).
There have been many studies that confirm the safety of FSS for stage I EOC patients. Histologic tumor grade is one of the most important risk factors of recurrence of EOC. In a recent comprehensive literature review of 1,150 patients who underwent FSS, 21 teams reported that there were 139 relapsed patients. The recurrence rate in patients with stage IA/IC of G1/G2 tumors (7–11%) was lower than that of patients with G3 tumors (23–29%) (6). G3 tumor is an independent risk factor and is associated with distant relapse and lower OS (6). In addition, it has been reported that although FSS can be safe and appropriate for patients with G1/G2 tumors, it is not recommended for patients with G3 tumors (10).
CCC has a great risk of recurrence and poor prognosis in comparison with other pathological types (serous, endometrioid, and mucinous) of EOC because of a relative resistance to first line platinum-based chemotherapy (11). According to the American College of Obstetricians and Gynecologists (ACOG) and ESMO guidelines, CCC is classified as an unfavorable histological type for FSS (5,12). In a recent study, G3 and CCC were the only independent risk factors for the survival of stage I EOC patients of reproductive age (13). It has been noted that the time to give pregnancy permission after FSS should be considered carefully, because the majority of reported recurrences of CCC occurred at extraovarian locations within the first two years following initial surgical staging (14). On the other hand, the selection criteria for FSS could include patients with stage IA CCC (15,16). There was no significant difference in the RFS and OS between the EOC patients with G1/G2 and those with G3/CCC in the current study (Fig. 1), and histological grading did not show prognostic factors. The reason for this could be that the number of subjects in our study was too small to evaluate the appropriateness of FSS for patients with poor differentiation and unfavorable histology. Therefore, FSS should be considered with caution in G3/CCC patients.
Staging is also an important factor for prognosis of EOC patients who undergo FSS. A previous study suggested that patients with stage IA EOC have survival approaching 94%, whereas it is approximately 84% for patients with IC EOC (17). FIGO staging represents a change of stage IC if cyst rupture is noted preoperatively or postoperatively. Although in patients with intraoperative rupture, survival was not different from patients whose tumors had intact capsules (85 and 78%, respectively), it was significantly different from patients with preoperative rupture (59%) (18). Recently, the 2014 FIGO staging system modified stage IC and subdivided it into three new clinically and prognostically more relevant subgroups (19). Although no significant survival differences in patients have been observed between stage IC1 and stage IA, the survival of stage IC2/3 has been reported to be worse than that of stage IA and IC1 (20,21). In a systematic review, the recurrence rate of stage IC2/3 (23%) after FSS was significantly higher than that of stage IC1 (12%) (14).
Although we were unable to investigate preoperative rupture in the current study because patients with IC2 were not included in the study population, cytology positive as stage IC3 indicated a high risk of recurrence in the patients who underwent FSS (Fig. 2A). Probably due to a small sample size, the recurrence rate in the IC3 and G3/CCC patients was very high (67%, 2 of 3; Table II). However, there was no significant difference in OS (Fig. 2B). Although the recurrence rate of stage IC3 (4/9) was more than that of stage IA/IC1 (1/20), the mortality rates were similar because three patients with recurrence in stage IC3 were curative. Further studies with larger sample sizes are needed to identify the differences in OS.
According to previous studies, recurrence in the remaining ovary is likely to be successfully treated with surgery and chemotherapy, and does not affect the long-term survival of FSS patients (14,22,23). However, patients who had relapse in LNs or PC, which is typical of clear cell histology, had a poor prognosis (24). Compared with G1/G2 tumors, G3 tumors give rise to a higher rate of extraovarian recurrences (6,14). In the current study, the recurrence sites were the pelvis (n=2), LN and peritoneum (n=1) and LN (n=2), and there were no patients showing recurrence exclusively in the residual ovary (Table II). The extraovarian recurrence rate of the G1/G2 tumors (3/23, 13%) was lower than that of the G3/CCC tumors (2/6, 33.3%), and this result was similar to that of a previous report (G1; 9%, G2; 11.2%, G3; 23.5%, CCC; 15.3%) (25). The extraovarian recurrence rate in patients with positive cytology (4/9, 44.4%) also showed a higher proportion than that in patients with negative cytology (1/20, 5%) in the present study.
The reproductive outcome was considered favorable in our series. Most patients had regular menstruation after surgery and chemotherapy. Four patients, who underwent chemotherapy, gave birth to six infants without congenital anomalies. Recent studies have described good reproductive outcomes after chemotherapy for malignant tumors (10,13). The usefulness of a wedge biopsy of the normal-appearing contralateral ovary in FSS is unknown. In recent data, an incidence of microscopic metastasis in the opposite normal looking ovaries was reported to be approximately 0–5% (10,26). However, none of the patients who underwent FSS had microscopic metastases in their macroscopically normal ovaries detected by routine biopsies (27,28). In another series, micrometastasis in an ovary with a grossly normal appearance was very rare (3 of 118, 2.5%) (29). The survival of patients with an isolated ovarian recurrence is better than that of patients with other types of recurrence (22). In the present study, among the five patients who had recurrence, none experienced said recurrence on the contralateral ovary. As wedge biopsy can cause mechanical infertility or ovarian failure, if the opposite ovary is of normal size, shape, and position, surgical evaluation should not be routinely performed.
There were some limitations in the present study. The first and biggest limitation is the small sample size, compared to those of other multicenter research studies. Survival evaluation of detailed classifications, including histological tumor grade, histology and staging, could not be performed. A larger sample size would likely provide more definitive results. Secondly, our study was not a randomized controlled trial, but a retrospective study, and did not have sufficient power to provide a definitive conclusion. Thirdly, although obstetric outcome is as important as oncological safety for patients receiving FSS, we unfortunately could not present data on reproductive outcome because some young patients who were married could not be followed up on for adequate periods and several unmarried patients were included in this study. Fourthly, we were unable to obtain the total number and information of all EOC patients in each institution. Therefore, we could not compare FSS and radical surgery for stage I EOC. Finally, lymphadenectomy was not performed for some patients because they had often undergone surgery for a presumed benign tumor or emergency. LN evaluation is recommended in the surgical treatment of early stage EOC according to the FIGO criteria, in order to perform accurate clinical staging and to select an adequate adjuvant systemic chemotherapy.
In conclusion, our data confirmed that FSS could be considered in the treatment of fertile women with stage IA and IC1 EOC and has a sufficient reproductive and obstetrical outcome. However, we were unable to confirm the safety of FSS for patients with stage IC3 EOC. Since the increased risk of stage I EOC in patients with positive cytology is mainly related to a higher incidence of extraovarian spread rather than to a higher relapse rate in the preserved ovary, these patients should be carefully informed about their prognosis when FSS is chosen. Our results are consistent with those of previous studies and we believe that these data can help treat early EOC in young women who wish to bear children following treatment. In future, prospective controlled studies should be conducted to confirm these findings.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Tohoku Gynecologic Cancer Unit (Morioka, Japan).
Availability of data and materials
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request
Authors' contributions
TW drafted the manuscript and analyzed the data. SSo analyzed the data. YK supervised the centralized pathological review. HN, HT, SSh, NY, HY, TO, SN, TS, MK, TB, DS, NS, YT, MF, YY and KF contributed to the study design and data collection. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Research Ethics Committee of each of the seven participating institutions belonging to the TGCU: Fukushima Medical University School of Medicine (Fukushima, Japan), Tohoku University Graduate School of Medicine (Sendai, Japan), Miyagi Cancer Center (Natori, Japan), Yamagata University School of Medicine (Yamagata, Japan), Iwate Medical University School of Medicine (Morioka, Japan), Akita University School of Medicine (Akita, Japan) and Hirosaki University School of Medicine (Hirosaki, Japan). All patients had provided written informed consent for surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Nasioudis D, Chapman-Davis E, Frey MK, Witkin SS, Holcomb K. Could fertility-sparing surgery be considered for women with early stage ovarian clear cell carcinoma? J Gynecol Oncol. 2017;28:e71. doi: 10.3802/jgo.2017.28.e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin J, Wang Y, Shan Y, Li Y, Jin Y, Pan L. Pregnancy and oncologic outcomes of early stage low grade epithelial ovarian cancer after fertility sparing surgery: A retrospective study in one tertiary hospital of China. J Ovarian Res. 2019;12:44. doi: 10.1186/s13048-019-0520-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eftekhar M, Pourmasumi S, Karimi-Zarchi M. Preservation of ovarian function during chemotherapy and radiotherapy in young women with malignancies. Iran J Reprod Med. 2014;12:377–382. [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan RJ, Jr, Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Behbakht K, Chen LM, Copeland L, Crispens MA, DeRosa M, Dorigo O, et al. Ovarian cancer, version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14:1134–1163. doi: 10.6004/jnccn.2016.0122. [DOI] [PubMed] [Google Scholar]
- 5.Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C, ESMO Guidelines Working Group Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi24–vi32. doi: 10.1093/annonc/mdt333. [DOI] [PubMed] [Google Scholar]
- 6.Fruscio R, Corso S, Ceppi L, Garavaglia D, Garbi A, Floriani I, Franchi D, Cantù MG, Bonazzi CM, Milani R, et al. Conservative management of early-stage epithelial ovarian cancer: Results of a large retrospective series. Ann Oncol. 2013;24:138–144. doi: 10.1093/annonc/mds241. [DOI] [PubMed] [Google Scholar]
- 7.Schlaerth AC, Chi DS, Poynor EA, Barakat RR, Brown CL. Long-term survival after fertility-sparing surgery for epithelial ovarian cancer. Int J Gynecol Cancer. 2009;19:1199–1204. doi: 10.1111/IGC.0b013e31819d82c3. [DOI] [PubMed] [Google Scholar]
- 8.Melamed A, Rizzo AE, Nitecki R, Gockley AA, Bregar AJ, Schorge JO, Del Carmen MG, Rauh-Hain JA. All-cause mortality after fertility-sparing surgery for stage I epithelial ovarian cancer. Obstet Gynecol. 2017;130:71–79. doi: 10.1097/AOG.0000000000002102. [DOI] [PubMed] [Google Scholar]
- 9.Ditto A, Martinelli F, Lorusso D, Haeusler E, Carcangiu M, Raspagliesi F. Fertility sparing surgery in early stage epithelial ovarian cancer. J Gynecol Oncol. 2014;25:320–327. doi: 10.3802/jgo.2014.25.4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JY, Kim DY, Suh DS, Kim JH, Kim YM, Kim YT, Nam JH. Outcomes of fertility-sparing surgery for invasive epithelial ovarian cancer: Oncologic safety and reproductive outcomes. Gynecol Oncol. 2008;110:345–353. doi: 10.1016/j.ygyno.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 11.Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, Suzuki M, Sato I, Taguchi K. Clinical characteristics of clear cell carcinoma of the ovary: A distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer. 2000;88:2584–2589. doi: 10.1002/1097-0142(20000601)88:11<2584::AID-CNCR22>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 12.American College of Obstetricians and Gynecologists: ACOG practice bulletin. Management of adnexal masses. Obstet Gynecol. 2007;110:201–214. doi: 10.1097/01.AOG.0000263913.92942.40. [DOI] [PubMed] [Google Scholar]
- 13.Jiang X, Yang J, Yu M, Xie W, Cao D, Wu M, Pan L, Huang H, You Y, Shen K. Oncofertility in patients with stage I epithelial ovarian cancer: Fertility-sparing surgery in young women of reproductive age. World J Surg Oncol. 2017;15:154. doi: 10.1186/s12957-017-1222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bentivegna E, Gouy S, Maulard A, Pautier P, Leary A, Colombo N, Morice P. Fertility-sparing surgery in epithelial ovarian cancer: A systematic review of oncological issues. Ann Oncol. 2016;27:1994–2004. doi: 10.1093/annonc/mdw311. [DOI] [PubMed] [Google Scholar]
- 15.Satoh T, Hatae M, Watanabe Y, Yaegashi N, Ishiko O, Kodama S, Yamaguchi S, Ochiai K, Takano M, Yokota H, et al. Outcomes of fertility-sparing surgery for stage I epithelial ovarian cancer: A proposal for patient selection. J Clin Oncol. 2010;28:1727–1732. doi: 10.1200/JCO.2009.24.8617. [DOI] [PubMed] [Google Scholar]
- 16.Kajiyama H, Shibata K, Mizuno M, Hosono S, Kawai M, Nagasaka T, Kikkawa F. Fertility-sparing surgery in patients with clear-cell carcinoma of the ovary: Is it possible? Hum Reprod. 2011;26:3297–3302. doi: 10.1093/humrep/der342. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed FY, Wiltshaw E, A'Hern RP, Nicol B, Shepherd J, Blake P, Fisher C, Gore ME. Natural history and prognosis of untreated stage I epithelial ovarian carcinoma. J Clin Oncol. 1996;14:2968–2975. doi: 10.1200/JCO.1996.14.11.2968. [DOI] [PubMed] [Google Scholar]
- 18.Sjövall K, Nilsson B, Einhorn N. Different types of rupture of the tumor capsule and the impact on survival in early ovarian carcinoma. Int J Gynecol Cancer. 1994;4:333–336. doi: 10.1046/j.1525-1438.1994.04050333.x. [DOI] [PubMed] [Google Scholar]
- 19.Prat J, FIGO Committee on Gynecologic Oncology Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014;124:1–5. doi: 10.1016/j.ijgo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Rosendahl M, Høgdall CK, Mosgaard BJ. Restaging and survival analysis of 4036 ovarian cancer patients according to the 2013 FIGO classification for ovarian, fallopian tube, and primary peritoneal cancer. Int J Gynecol Cancer. 2016;26:680–687. doi: 10.1097/IGC.0000000000000675. [DOI] [PubMed] [Google Scholar]
- 21.Suh DH, Kim TH, Kim JW, Kim SY, Kim HS, Lee TS, Chung HH, Kim YB, Park NH, Song YS. Improvements to the FIGO staging for ovarian cancer: Reconsideration of lymphatic spread and intraoperative tumor rupture. J Gynecol Oncol. 2013;24:352–358. doi: 10.3802/jgo.2013.24.4.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zapardiel I, Diestro MD, Aletti G. Conservative treatment of early stage ovarian cancer: Oncological and fertility outcomes. Eur J Surg Oncol. 2014;40:387–393. doi: 10.1016/j.ejso.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 23.Marpeau O, Schilder J, Zafrani Y, Uzan C, Gouy S, Lhommé C, Morice P. Prognosis of patients who relapse after fertility-sparing surgery in epithelial ovarian cancer. Ann Surg Oncol. 2008;15:478–483. doi: 10.1245/s10434-007-9651-x. [DOI] [PubMed] [Google Scholar]
- 24.Groen RS, Gershenson DM, Fader AN. Updates and emerging therapies for rare epithelial ovarian cancers: One size no longer fits all. Gynecol Oncol. 2015;136:373–383. doi: 10.1016/j.ygyno.2014.11.078. [DOI] [PubMed] [Google Scholar]
- 25.Bentivegna E, Fruscio R, Roussin S, Ceppi L, Satoh T, Kajiyama H, Uzan C, Colombo N, Gouy S, Morice P. Long-term follow-up of patients with an isolated ovarian recurrence after conservative treatment of epithelial ovarian cancer: Review of the results of an international multicenter study comprising 545 patients. Fertil Steril. 2015;104:1319–1324. doi: 10.1016/j.fertnstert.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Morice P, Wicart-Poque F, Rey A, El-Hassan J, Pautier P, Lhommé C, de Crevosier R, Haie-Meder C, Duvillard P, Castaigne D. Results of conservative treatment in epithelial ovarian carcinoma. Cancer. 2001;92:2412–2418. doi: 10.1002/1097-0142(20011101)92:9<2412::AID-CNCR1590>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 27.Morice P, Camatte S, El Hassan J, Pautier P, Duvillard P, Castaigne D. Clinical outcomes and fertility after conservative treatment of ovarian borderline tumors. Fertil Steril. 2001;75:92–96. doi: 10.1016/S0015-0282(00)01633-2. [DOI] [PubMed] [Google Scholar]
- 28.Zanetta G, Chiari S, Rota S, Bratina G, Maneo A, Torri V, Mangioni C. Conservative surgery for stage I ovarian carcinoma in women of childbearing age. Br J Obstet Gynaecol. 1997;104:1030–1035. doi: 10.1111/j.1471-0528.1997.tb12062.x. [DOI] [PubMed] [Google Scholar]
- 29.Benjamin I, Morgan MA, Rubin SC. Occult bilateral involvement in stage I epithelial ovarian cancer. Gynecol Oncol. 1999;72:288–291. doi: 10.1006/gyno.1998.5260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request