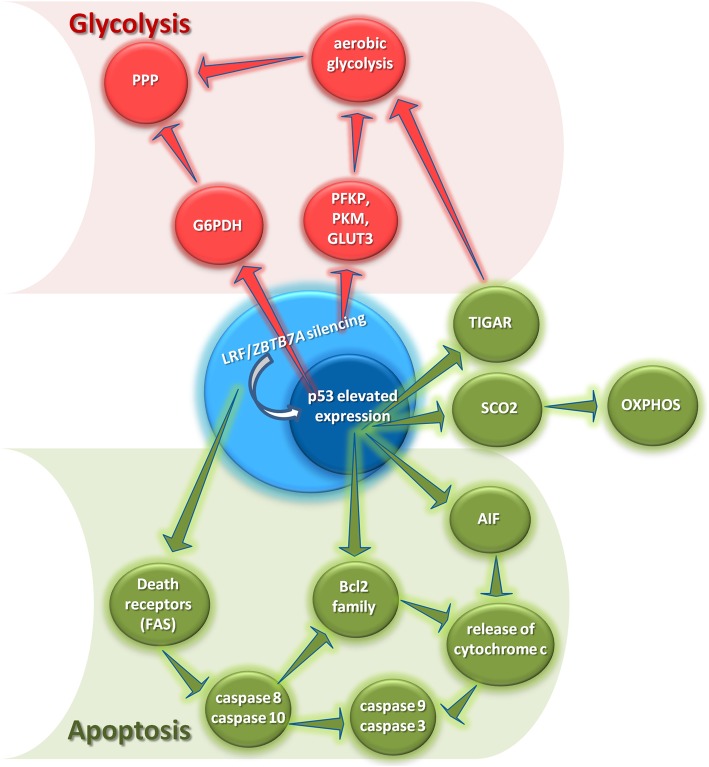
Fig. 2.
LRF/ZBTB7A’s silencing compromises Warburg effect and induces apoptosis in cancer cells. The inhibition of aerobic glycolysis and concomitant activation of oxidative phosphorylation (OXPHOS) is promoted by the LRF/ZBTB7A’s silencing and the subsequent activation of the expression and phosphorylation of p53, leading thus to compromised Warburg effect in cancer cells. Furthermore, LRF/ZBTB7A’s silencing induces apoptosis in cancer cell lines by mediating both known apoptotic pathways (intrinsic and extrinsic) as well as their cross-talk. That is, upon LRF/ZBTB7A’s silencing and the subsequent activation of p53, the pro-apoptotic Bcl-2 family proteins as well as AIF are activated and promote cytochrome c release from mitochondria and subsequent activation of caspase-9 and caspase-3 (intrinsic apoptotic pathway). Meanwhile, LRF/ZBTB7A’s silencing promotes the expression of the Fas receptor (death receptor), leading to the activation of the downstream caspase-10 and caspase-8 and leading thus to activation of the extrinsic apoptotic pathway. In addition, caspace-8 further enhances the activation of Bcl2 family members, as well as the activation of caspase-9 and caspase-3 supporting the hypothesis that LRF/ZBTB7A, besides affecting both apoptotic pathways, potentially mediates the cross-talk between the intrinsic and extrinsic apoptotic pathways in cancer cells (in red color are cellular processes inhibited and in green color are cellular processes activated by LRF/ZBTB7A silencing). Abbreviations: AIF, apoptosis inducing factor; FasR, Fas receptor; G6PDH, glucose-6-phosphate dehydrogenase; OXPHOS, oxidative phosphorylation; PPP, pentose phosphate pathway; SCO2, cytochrome c oxidase 2; TIGAR, TP53-induced glycolysis and apoptosis regulator