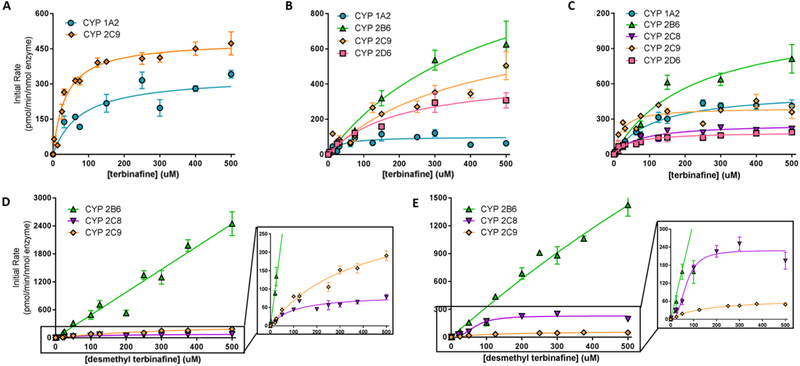
Fig. 2. Steady state kinetic profiles for N-dealkylation primary and secondary metabolites of terbinafine in recombinant P450s.
N-Dealkylation of terbinafine in CYP1A2 (blue circle), 2B6 (green triangle), 2C8 (purple inverted triangle), 2C9 (orange diamond), and 2D6 (pink square) yielded metabolic kinetics for one to three primary metabolites. The kinetic profiles include those for (A) TBF-A (dansyl hydrazine labeled) and (B) N-methyl-1-naphthyl methylamine (dansyl chloride labeled) from Pathway 1, and (C) desmethyl-terbinafine (dansyl chloride labeled) from Pathway 2. Secondary metabolites of terbinafine were formed from metabolism of desmethyl-terbinafine in Pathway 2, yielding (D) 1-naphthyl methylamine and (E) 1-naphthaldehyde. All sets of data were fit best to the Michaelis-Menten equation (p < 0.05), and the corresponding constants reported in Table 1. Six to nine experimental reactions were carried out and analyzed as described in Materials and Methods.