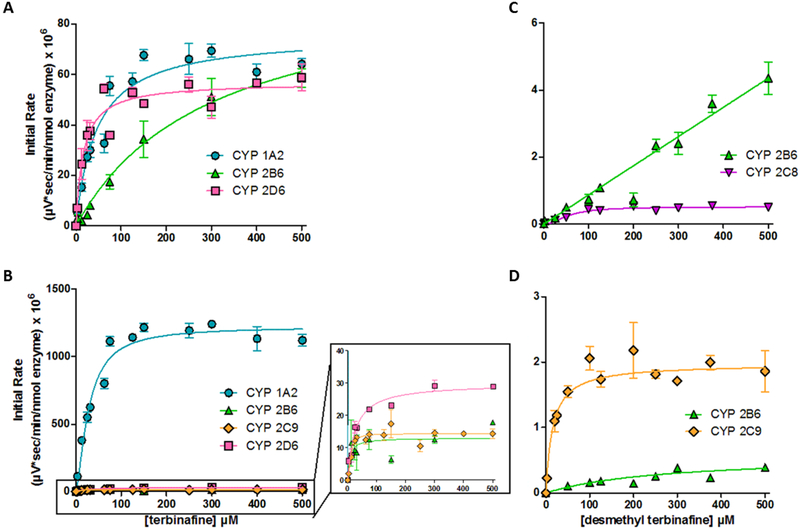
Fig. 3. Steady state kinetic profiles for metabolites of major oxidative non-N-dealkylation pathways of terbinafine in recombinant P450s.
Metabolites for major pathways (Vickers et al., 1999) (Kovarik et al., 1995) competing with N-dealkylation. (A) Hydroxyterbinafine and (B) terbinafine dihydrodiol from terbinafine, and (C) desmethyl hydroxyterbinafine and (D) desmethyl-terbinafine dihydrodiol from desmethyl-terbinafine were observed for CYP1A2 (blue circle), 2B6 (green triangle), 2C9 (orange diamond), and 2D6 (pink square). Compounds were not quantitated due to unavailability of standards, so initial rates are normalized to MS peak areas as a function of time. Data were fit best to the Michaelis-Menten equation or the Hill equation (p < 0.05), and the corresponding constants reported in Table 2. At least six experimental reactions per condition were carried out and analyzed as described in Materials and Methods.