To the Editor,
Twenty-five percent of acne vulgaris patients are prescribed oral antibiotic courses lasting >6 months(Barbieri et al. 2017). These courses can cause significant “collateral damage” (dysbiosis, antibiotic resistance)(Leyden et al. 2014). Acne antibiotics target Cutibacterium (formerly Propionibacterium) acnes(Scholz and Kilian 2016). This bacterium is associated with acne and can cause opportunistic infections(Achermann et al. 2014).
Though antibiotics improve acne, the only “cure” is isotretinoin, a non-antimicrobial(Weissmann et al. 1981), systemic retinoid. While an isotretinoin course improves most acne patients, 10–20% of patients require retreatment(Leyden et al. 2014). Sebum and C. acnes decrease during treatment and then recover to pre-treatment levels(Leyden and McGinley 1982), while acne remission frequently persists for life. As such, our understanding of isotretinoin’s mechanism remains unclear.
The post-pubertal lipid-rich pilosebaceous unit (PSU) is dominated by C. acnes communities (1–13 strains/person)(Fitz-Gibbon et al. 2013; Scholz et al. 2014). Specific C. acnes strains and genomic elements have been associated with normal skin and others associated with acne(Fitz-Gibbon et al. 2013; Barnard et al. 2016). Until recently though, no studies had investigated the microbial community response to acne therapy.
In 2017, two groups reported that short-term acne treatments (6–10 weeks, antimicrobial and non-antimicrobial) changed the facial skin microbiome (Coughlin et al. 2017; Kelhälä et al. 2017). Neither study examined patients after acne remission or discontinuing treatment. In parallel with these groups, we have been pursuing work focused on answering these questions.
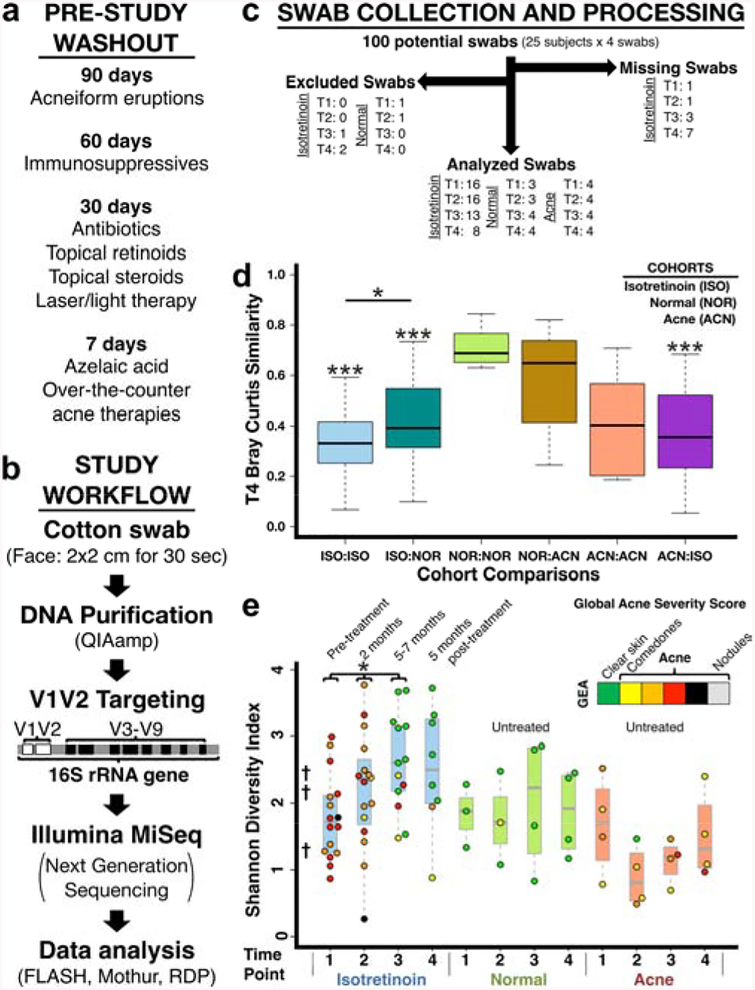
We hypothesized that successful acne treatment with isotretinoin would shift an “acne microbiome” to resemble that found in normal skin. Over ~2 years in St. Louis, MO, we performed a pilot, observational, prospective study comparing isotretinoin-treated acne subjects to two untreated controls groups (normal skin, acne) (Table S1). There were no significant differences in demographics between groups. Exclusion criteria were history of non-acne facial rash or pre-study exposures that might impact acne, immunity, or microbes (Figure 1a). Isotretinoin subjects were evaluated before (time point 1: T1, 0 months), during (T2, 2 months: T3, 5–7 months), and after (T4, +5 months) treatment. Controls were evaluated at 0, 2, 5, and 10 months. Acne severity (GEA score), skin sampling, control samples, targeted metagenomic next-generation sequencing (16S rRNA gene V1-V2 hypervariable region), and genomic analyses were performed as previously described (Figure 1b) (Fitz-Gibbon et al. 2013; Grice et al. 2009; Kong et al. 2017). See Supplemental Methods for further details.
Figure 1. Study design, workflow, processing, and comparison to prior studies.
(a) Prohibited exposures listed by washout time. “Acneiform eruptions” refers to any medication/exposure reported to cause this presentation. (b) Study workflow from swab collection to genomic analysis. DDBJ/EMBL/GenBank Accession KCKB01000000. (c) Flow diagram of study swabs. Missing Swabs: not available due to subjects missing visits. Excluded Swabs (low quality swabs): poor 16S amplification (<5 ng/μl by qPCR) and/or low classified 16S sequence count (<2500). Swab numbers are listed by cohort and time point. (d) Post-treatment beta-diversity is significantly increased (i.e. decreased similarity) for isotretinoin subjects. (e) Alpha-diversity increases during treatment, remains elevated, and parallels the changing skin environment due to isotretinoin (sebaceous to dry). †Average Shannon diversity of prior Human Microbiome Project (HMP) study (Grice – sebaceous: 1.2, wet: 2.2, dry: 2.3). Data colored by acne severity (GEA). p-value<*0.05,***<0.001.
We designed our sampling protocol to be integrated into a routine, 5–15 minute acne visit. Subjects were sampled as they presented to clinic (i.e. no standardized skin hygiene regimen) with only minimal pre-study washout (Figure 1a). We swabbed active facial acne or malar cheek if no acne was present. Swabs were obtained rapidly (<1 minute), as compared to follicular caste sampling of the PSU (10–15 minutes). Swab microbiome data mirrors PSU data and can be used for C. acnes-related characterization(Hall et al. 2018). All available skin swabs (88) from 17 isotretinoin-treated subjects and 8 untreated controls (4 normal skin, 4 acne) underwent initial processing (Figure 1c, Tables S2–3).
Samples with sufficient microbial DNA (83 swabs, Figures 1c; S1) were first evaluated on a community level. Bray-Curtis taxa clustering correlated with acne severity and isotretinoin treatment (Figure S2). Compared to treatment naïve subjects, normal subjects and isotretinoin subjects with prior isotretinoin exposure had significantly more similar communities (decreased beta-diversity) prior to therapy (Figure S3–4). Following therapy, isotretinoin subjects had significantly increased beta-diversity (Figure 1d). Alpha-diversity increased significantly as a function of isotretinoin treatment time and remained elevated 5 months after treatment (Figures 1e; S5) significantly extending a recent report at 6 weeks(Kelhälä et al. 2017). Increased alpha-diversity may reflect isotretinoin-mediated changes in the skin microenvironment (sebaceous→dry). Subgroup analysis of our subjects with prior isotretinoin exposure (>1 year prior) suggests that this increased alpha-diversity is temporary (Figure S6).
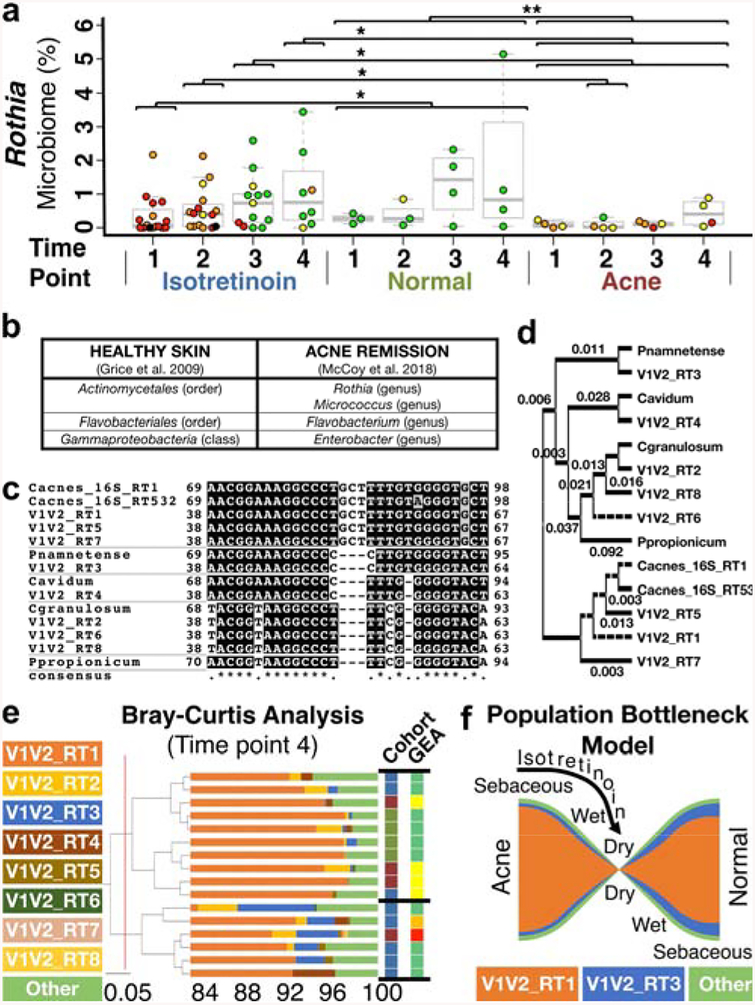
On a genus level, our culture-independent genomic approach now validates the prior suggestion of culture-dependent studies that C. acnes globally declines throughout treatment (Figure S7a). We also observed that Staphylococcal spp. increase initially but then decrease prior to completing treatment (Figure S7b), an observation not previously reported but in line with prior assessments of dry skin(Grice et al. 2009). Four taxa not previously associated with acne remission increase during therapy and remain elevated (Figures 2a–b; S7c–e, Table S4 – Rothia, Flavobacterium, Enterobacter, Micrococcus). Interestingly, isotretinoin T1 appears distinct from the controls (Figure 2a; S7) possibly due to prior acne treatments.
Figure 2. Skin microbiome community changes during isotretinoin therapy.
(a) Swarm plot showing significantly increased Rothia in normal controls and as a function of treatment/remission. Data colored by GEA. (b) Correlation of previously identified healthy sebaceous skin taxa with acne remission-associated taxa. (c-e) Eight dominant Propionibacterium genus V1V2_RTs were identified (Figures S8–9) with a median of 4 per subject (range 1–7), as compared to prior work on distinct 16S C. acnes RTs (Table S9, Figure S10) with 3±2 ribotypes/subject(Fitz-Gibbon et al. 2013). (c) T-Coffee alignment and (d) phylogenetic tree of V1-V2 demonstrating sub-taxa discrimination (C. acnes, granulosum, avidum; Propionibacterium namnetense; Pseudopropionibacterium propionicum). (e) V1V2_RT3 increases during and after treatment (Figure S11c) leading to Bray-Curtis clustering. Cohort indicated by text color (a). (f) Proposed model for isotretinoin-mediated remodeling of Propionibacterium communities through skin environment modulation.
While Propionibacterium genus globally decreases during treatment, there appears to be varying effects on sub-taxa within this genus. Our V1-V2 hypervariable region analysis suggests that Propionibacterium genus de novo V1-V2 ribotypes (V1V2_RTs) can differentiate four different Propionibacterium species, as well as three C. acnes subspecies (Figures 2c–d, S8–10; SUPPLEMENTAL RESULTS, Tables S8–9). V1V2_RT3, one of the eight most abundant V1V2_RTs, trends upward during and after treatment (Figures S11c, 2e), as compared to acne-associated V1V2_RT6 (Figure S11e). Further, a majority of our subjects had a distinct Propionibacterium community emerge during and then remain after isotretinoin treatment (low Propionibacterium – Figure S7a; high V1V2_RT3 – Figures 2e, S11c). The proportion of the remaining subjects (38%) is similar to that reported for isotretinoin subjects requiring future oral acne therapy (antibiotics/isotretinoin). We suggest that isotretinoin creates a Propionibacterium “population bottleneck” that selects for “healthy” Propionibacterium communities (Figure 2f) and other sebaceous skin taxa that persist after treatment resulting in long term acne remission (i.e. normal skin).
This investigation confirmed prior work and greatly expands our understanding of acne pathogenesis by identifying microbial features of isotretinoin-mediated acne remission, yet many questions still remain. Do “healthy” skin taxa (e.g. V1V2_RT3, Rothia) promote clear skin or are they only correlated with a clinical phenotype? If they are involved, when must they be present (before, during, or after therapy), where do they reside, and how long must they remain? Do post-treatment Propionibacterium communities predict the need for future treatments? We hope that our work will inspire future studies to answer these questions and ultimately lead to the development of “prebiotic fertilizers”, strain-selective “weed killers,” and/or probiotic Propionibacterium strains that optimize PSU ecosystems while avoiding antibiotic “collateral damage”.
Supplementary Material
Acknowledgements:
This study was funded through a 2015 American Acne and Rosacea Society Clinical Research Grant. Author support during these studies also included the Oliver Langenberg Physician Scientist Training Pathway (McCoy) and Washington University MA/MD Research Program (Otchere).
Abbreviations:
- FLASH
fast length adjustment of short reads
- GEA
Global Evaluation Acne
- HMP
Human Microbiome Project
- LEfSe
linear discriminant analysis effect size
- PSU
pilosebaceous unit
- RDP
Ribosomal Database Project
- rRNA
ribosomal ribonucleic acid
- RT
ribotype
- T1
time point 1
- T2
time point 2
- T3
time point 3
- T4
time point 4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
Ms. Otchere, Mr. Martin, and Drs. McCoy, Rosa, Mann, and Mitreva have no conflicts of interest.
References:
- Achermann Y, Goldstein EJC, Coenye T, Shirtliff ME. Propionibacterium acnes: from Commensal to Opportunistic Biofilm-Associated Implant Pathogen. Clin. Microbiol. Rev 2014;27(3):419–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: A retrospective analysis, 2004–2013. J. Am. Acad. Dermatol 2017;77(3):456–463.e4 [DOI] [PubMed] [Google Scholar]
- Barnard E, Shi B, Kang D, Craft N, Li H. The balance of metagenomic elements shapes the skin microbiome in acne and health. Sci. Rep 2016;6(1) Available from: http://www.nature.com/articles/srep39491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin CC, Swink SM, Horwinski J, Sfyroera G, Bugayev J, Grice EA, et al. The preadolescent acne microbiome: A prospective, randomized, pilot study investigating characterization and effects of acne therapy. Pediatr. Dermatol. 2017; Available from: http://doi.wiley.com/10.1111/pde.13261 [DOI] [PubMed] [Google Scholar]
- Fitz-Gibbon S, Tomida S, Chiu B-H, Nguyen L, Du C, Liu M, et al. Propionibacterium acnes Strain Populations in the Human Skin Microbiome Associated with Acne. J. Invest. Dermatol 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, et al. Topographical and Temporal Diversity of the Human Skin Microbiome. Science. 2009;324(5931):1190–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JB, Cong Z, Imamura-Kawasawa Y, Kidd BA, Dudley JT, Thiboutot DM, et al. Isolation and Identification of the Follicular Microbiome: Implications for Acne Research. J. Invest. Dermatol 2018; Available from: http://linkinghub.elsevier.com/retrieve/pii/S0022202X18302240 [DOI] [PubMed] [Google Scholar]
- Kelhälä H-L, Aho VTE, Fyhrquist N, Pereira PAB, Kubin ME, Paulin L, et al. Isotretinoin and lymecycline treatments modify the skin microbiota in acne. Exp. Dermatol 2017; [DOI] [PubMed] [Google Scholar]
- Kong HH, Andersson B, Clavel T, Common JE, Jackson SA, Olson ND, et al. Performing Skin Microbiome Research: A Method to the Madness. J. Invest. Dermatol 2017;137(3):561–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J. Clin. Aesthetic Dermatol 2014;7(2 Suppl):S3. [PMC free article] [PubMed] [Google Scholar]
- Leyden JJ, McGinley KJ. Effect of 13-cis-retinoic acid on sebum production and Propionibacterium acnes in severe nodulocystic acne. Arch. Dermatol. Res 1982;272(3–4):331–7 [DOI] [PubMed] [Google Scholar]
- Scholz CFP, Jensen A, Lomholt HB, Brüggemann H, Kilian M. A Novel High-Resolution Single Locus Sequence Typing Scheme for Mixed Populations of Propionibacterium acnes In Vivo. Mokrousov I, editor. PLoS ONE. 2014;9(8):e104199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz CFP, Kilian M. The natural history of cutaneous propionibacteria, and reclassification of selected species within the genus Propionibacterium to the proposed novel genera Acidipropionibacterium gen. nov., Cutibacterium gen. nov. and Pseudopropionibacterium gen. nov. Int. J. Syst. Evol. Microbiol 2016;66(11):4422–32 [DOI] [PubMed] [Google Scholar]
- Weissmann A, Wagner A, Plewig G. Reduction of bacterial skin flora during oral treatment of severe acne with 13-cis retinoic acid. Arch. Dermatol. Res 1981;270(2):179–83 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.