Figure 3:
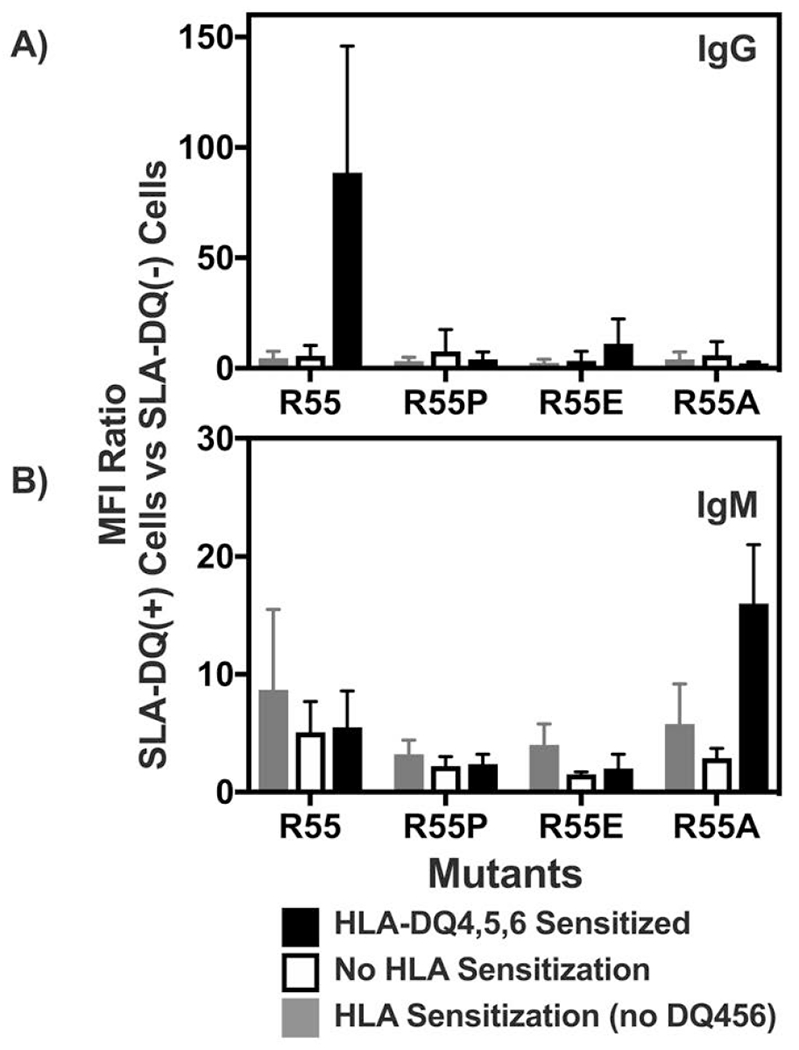
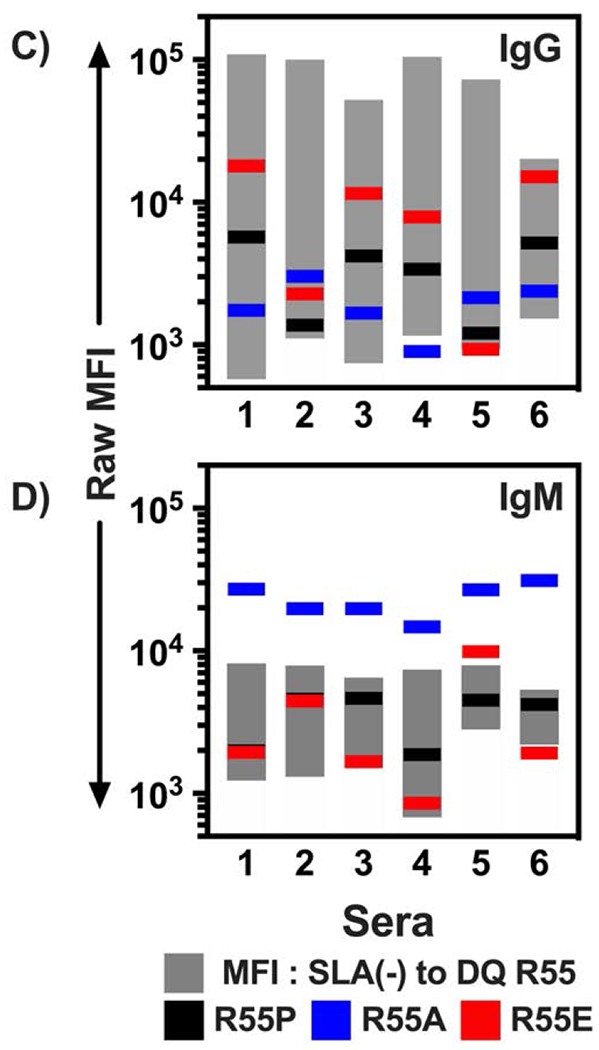
Flow cytometric analysis of human immunoglobulin binding to heterodimers of SLA-DQα*0101 and SLA-DQβ1*0601. The SLA-DQβ chain was mutated at position 55 to change arginine to either proline (R55P), alanine (R55A), or glutamate (R55E). Panels A and B: Human sera having no HLA sensitization (n=6, white bars), class II HLA sensitization, but not towards HLA-DQ4,5,6 (n=10; gray bars), or class II sensitization against HLA-DQ4,5,6 (n=6, black bars) were tested for reactivity with cells expressing individual SLA-DQ variants. Human IgG (panel A) and IgM (panel B) binding to each SLA-DQ variant was evaluated by flow cytometry. MFI of antibody binding to SLA-DQ expressing cells was divided by the MFI of the identical sample binding to cells lacking SLA-DQ expression (MFI Ratio). Means and standard deviations are shown. HLA-DQ456 sensitized sera exhibited high levels of IgG that reacted with cells expressing the R55 variant of SLA-DQ. All three mutants of amino acid 55 significantly reduced IgG binding (R55 vs R55P, p = 0.047; R55 vs R55E, p = 0.047, and R55 vs R55A, p = 0.048). IgM binding results were similar across the different sera and mutants with one exception. In HLA-DQ456 sensitized sera, the R55A mutant increased IgM binding to SLA-DQ (R55 vs R55A, p = 0.001). Panels C and D show the raw MFI of IgG (panel C) and IgM (panel D) binding from each of the six HLA-DQ456 sensitized sera to all four variants of SLA-DQ and to negative control cells that are SLA deficient. The bottom of the gray bar represents the MFI of each sample binding to the class II SLA-deficient cells, and the top of the gray bar represents the binding to cells expression the R55 variant of SLA-DQ. The data in panels A and B were analyzed using one-way ANOVA and Dunnet’s correction for multiple comparisons.
