Figure 4:
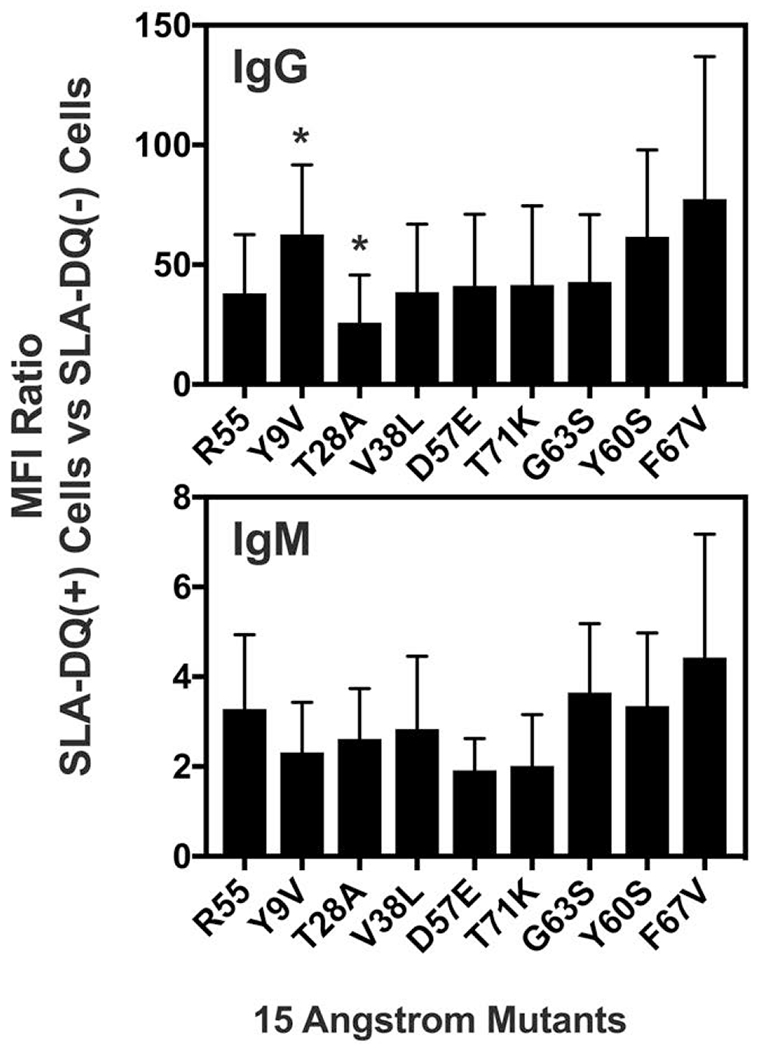
Binding of human antibodies to SLA-DQα*0101 and SLA-DQβ1*0601 heterodimers having beta chain point mutations within 15 angstroms of residue 55. Specific amino acid changes are noted as x-axis labels with as the single letter abbreviation of the original amino acid-the residue number-the mutant amino acid. Distances of each residue from position 55 are listed in table 2. Human IgM and IgG binding to cells expressing each variant of SLA-DQ were tested by flow cytometry with raw MFI values shown. Sera samples were collected from people having been sensitized to HLA-DQ456. These are the same samples tested in Fig 3. Cells devoid of SLA were used as negative controls. MFI ratios of IgG or IgM binding were SLA-DQ expressing cells vs SLA deficient cells were calculated. Means and standard deviations of these ratios for the six sera are shown. One-way Anova with Dunnet’s correction for multiple comparisons showed that the Y9V caused slight but significant elevations in IgG binding relative to the R55 SLA-DQ variant (p = 0.002). The T28A mutant slightly reduced IgG binding relative to the R55 SLA-DQ molecule (p = 0.020). All other IgG and IgM binding alterations did not reach statistical significance (p>0.05).
