Figure 1.
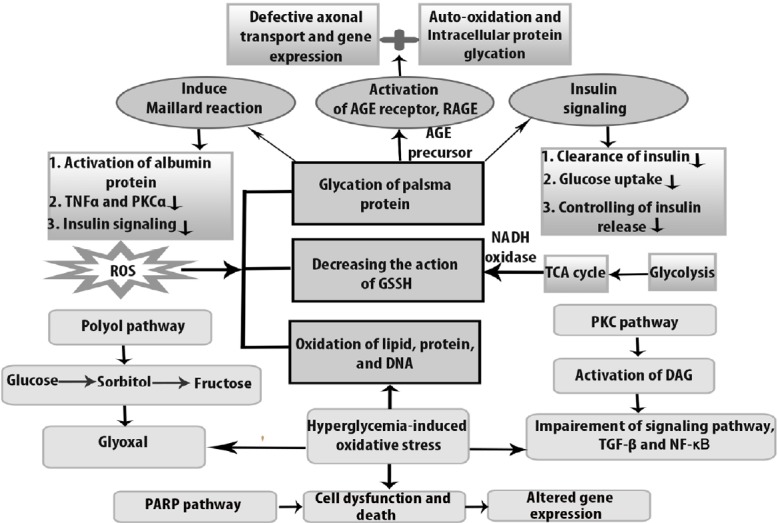
Oxidative stress-induced dysfunctional pathways in diabetic peripheral neuropathy.
The figure illustrates about the mechanism of cells of diabetic peripheral neuropathy through different signaling pathways, which lead to different abnormalities under oxidative stress condition. Hyperglycemia initiates dysfunction by producing metabolite glyoxal by which polyol pathway inhibits cytosolic nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) as well as glutathione (GSH). In the presence of excessive amount of glucose, advanced glycation end products (AGEs) bind with receptor for advanced glycation end products (RAGE) and impairs the activity of functional protein and causes auto-oxidation in diabetic peripheral neuropathy. As a consequence, insulin signaling is downregulated, whereas reactive oxygen species (ROS) acts as a second messenger. At the same time, due to maillard reaction, particularly albumin protein is depleted and tumor necrosis factor (TNFα) and protein kinase C alpha (PKCα) show less activity. PKC activation increases the hyperglycemic level and accelerates dysfunction in transcriptional factor, through nuclear factor kappa B (NF-κB) impairment. On the other hand, hyperglycemia-induced oxidative stress causes necrosis and abnormal gene expression through PARP pathway. GSSH: Oxidized glutathione; TCA: tricarboxylic acid cycle; TGF: transforming growth factor.
