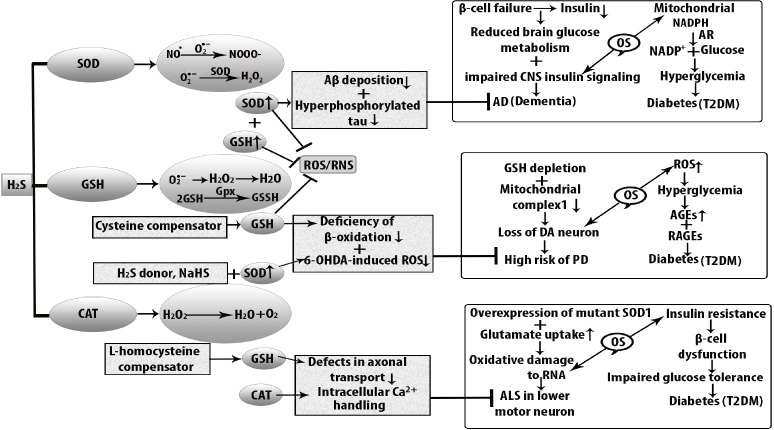
Figure 3.
Antioxidant function of H2S against oxidative stress in diabetes-induced neurodegenerative diseases.
Oxidative stress increases cellular dysfunction in type 2 diabetes mellitus (T2DM), Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (ALS). At the stage of hyperglycemia and in the early stage of insulin resistance, AD develops because of β cell failure, and lower level of insulin shows impaired central nervous system (CNS) signaling. As a consequence, brain glucose metabolism becomes reduced and neurodegenerative disease AD develops. Insulin resistance is also associated with the elevated level of amyloid beta (Aβ) accumulation and hyperphosphorylation of tau protein which are decreased by increased level of superoxide dismutase (SOD) in the mitochondria. Similarly, oxidative stress is responsible for diminishing nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) in T2DM. On the other hand, reactive oxygen species (ROS) generates in substantia nigra compacta. The level of DA neurons is higher than that of other neurons because oxidative metabolism of dopamine (DA) takes place in axon terminal. Antioxidant function of H2S is mediated by increased level of SOD which suppresses the 6-hydroxydopamine (6-OHDA)-induced ROS formation that ultimately reduces the risk of PD. At the same time, H2S donor, sodium hydrogen sulfide (NaHS) increases β-cell oxidation to decrease dopaminergic loss but reduced glutathione (GSH) is produced weakly in DA neurons. In the case of ALS, oxidative damage to ribonucleic acid (RNA) is seen in the CNS and SOD1 is over-expressed in mutant SOD1G93A. CAT: Catalase; H2S: hydrogen sulfide; OS: oxidative stress; RNS: reactive nitrogen species.