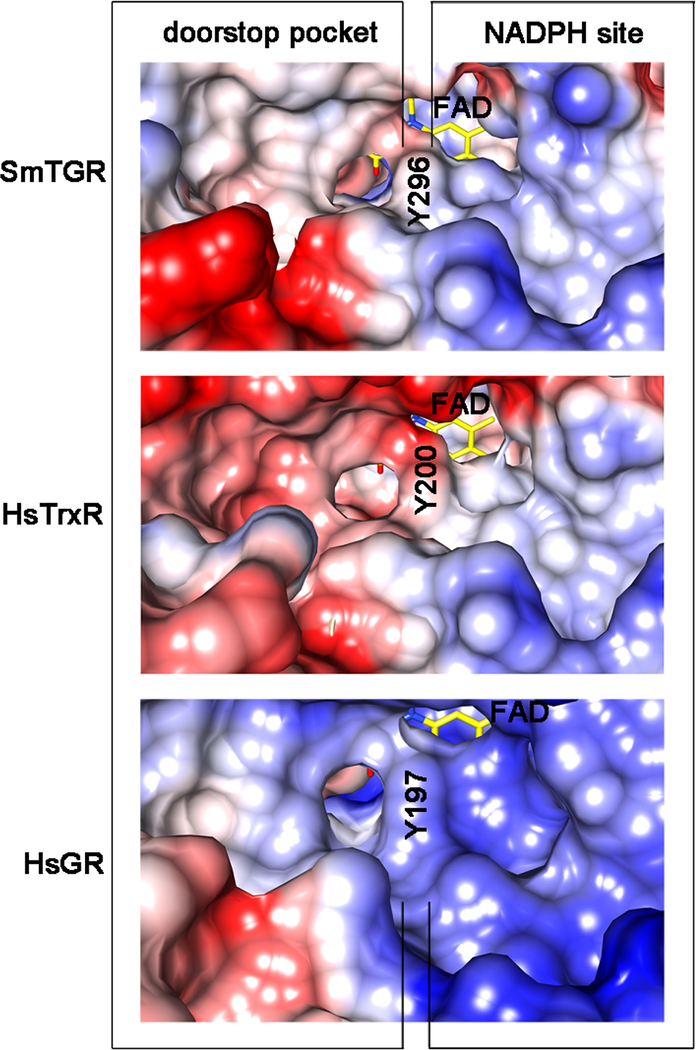
Figure 5.
Coulombic surfaces of SmTGR (at the top), human hTrxR1 (HsTrxR1, in the middle; pdb ID: 2CFY) and human GR (HsGR, at the bottom; pdb code: 3DK8) around the re-face of the FAD (in yellow sticks) calculated with the program CHIMERA are shown. The surface of the conserved tyrosine residue (Y296 in SmTGR, Y200 in HsTrxR 1 and Y197 in HsGR) is indicated in the three enzymes occupying the same relative position, i.e., pointing with its phenolic oxygen towards the isoalloxazine of the FAD and being at the boundary between the NADPH and the doorstop pocket. The NADPH binding sites on the right of the tyrosine are characterized by an overall positive charge (blue), necessary to bind the phosphates of the cofactor. The doorstop pockets in SmTGR, HsTrxR and HsGR, on the left, present different electrostatic and shape features.