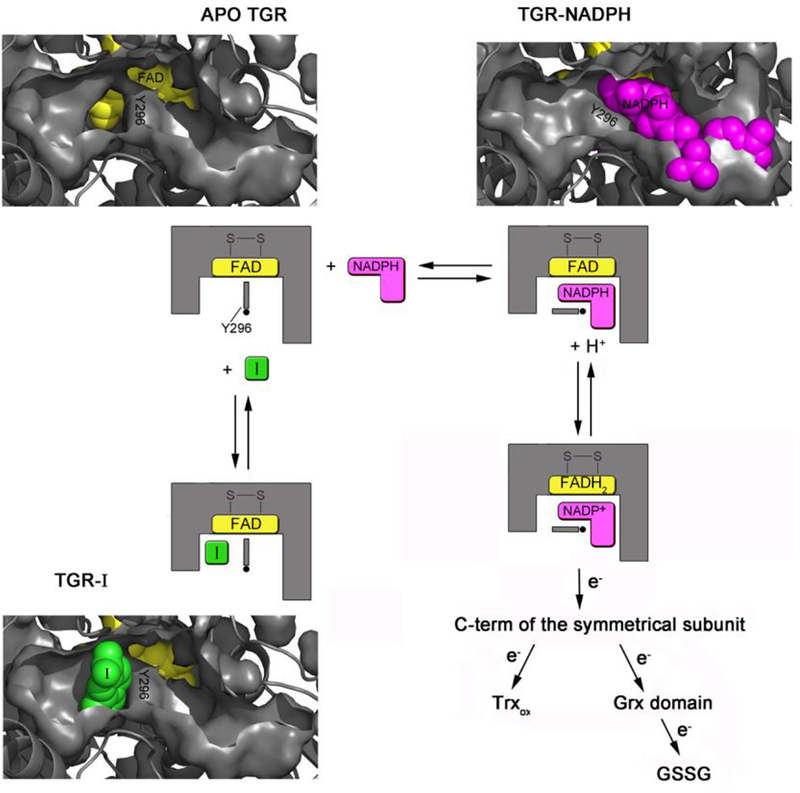
Figure 6.
Cartoon representation showing the proposed inhibition mechanism as assessed by both the structural superposition between the relative SmTGR complexes and steady state experiments. In one subunit of the dimeric unliganded TGR (in gray, pdb ID: 2V6O) two pockets, one on the left (the doorstop pocket) and one on the right of Y296 (the NADPH pocket), facing the isoalloxazine ring of the FAD (in yellow spheres) are present. Upon NADPH binding (NADPH is in magenta spheres, pdb ID of the TGR-NADPH complex: 2X99) the right pocket is filled by the cofactor, while the doorstop pocket completely disappears due to a conformational switch of Y296, necessary to make room to the nicotinamide moiety of the reductant. In the presence of 1 (in green spheres, at the bottom of the figure) the doorstop pocket is occupied hampering the tyrosine switch and thus enzyme reduction and turn-over. Several intermediates of the oxidative half reaction are omitted for simplicity (See Angelucci et al., 2010 and Prast-Nielsen et al., 2011 for a comprehensive description of the TGR mechanism).