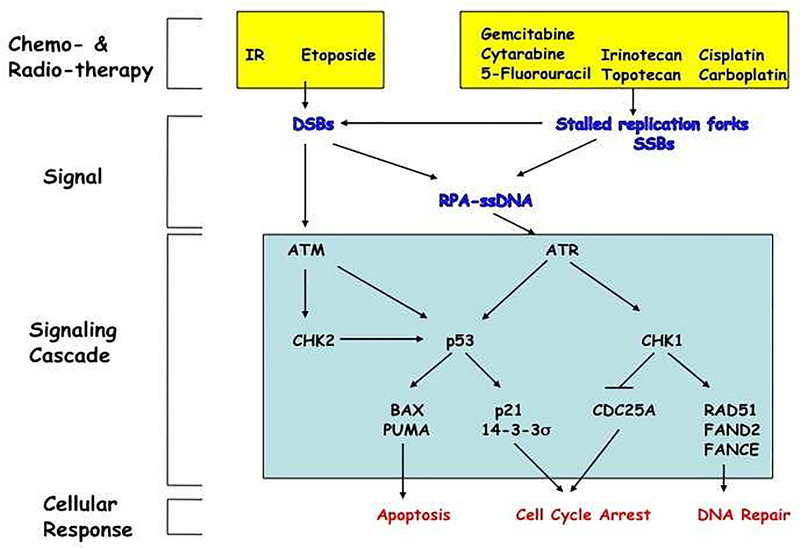
Figure 2. DNA damage response pathway.
Exposure of cells to IR or etoposide induces double strand breaks (DSBs) in DNA whereas exposure of cells to various chemotherapeutic agents (irinotecan, topotecan, cisplatin, carboplatin) or antimetabolites (gemcitabine, 5-fluorouracil, cytarabine) results in replication fork stalling and the generation of single strand breaks (SSBs). This, in turn, activates checkpoints that mobilize DNA repair pathways and either signals to the cell cycle machinery to prevent progression or induces apoptosis. ssDNA becomes coated with replication protein A (RPA), which recruits ATR as well as additional proteins thereby leading to full ATR activation. ATR phosphorylates many intracellular substrates including p53 and CHK1. ATR phosphorylates CHK1 on serines 317 and 345 resulting in CHK1 autophosphorylation on serine 296. Activated CHK1, in turn, phosphorylates the Cdc25A protein phosphatase to promote its ubiquitin-mediated proteolysis. Loss of Cdc25A results in cell cycle arrest in the S- and G2-phases of the cell division cycle. CHK1 also phosphorylates RAD51, FAND2 and FANCE to activate DNA repair pathways. DSBs activate ATM, which in turn phosphorylates both CHK2 and p53. p53 is also phosphorylated by CHK2. This leads to p53 accumulation and activation of its downstream target genes. Transcriptional activation of genes encoding BAX and PUMA lead to apoptosis whereas transcriptional activation of genes encoding p21 and 14–3-3s lead to G1 cell cycle arrest and also function to enforce the S- and G2-cell cycle arrests regulated by CHK1. Crosstalk exists between these pathways as stalled replication forks can lead to DSBs leading to ATM activation and the repair of DSBs can produce RPA-coated ssDNA that activates that ATR pathway [88].