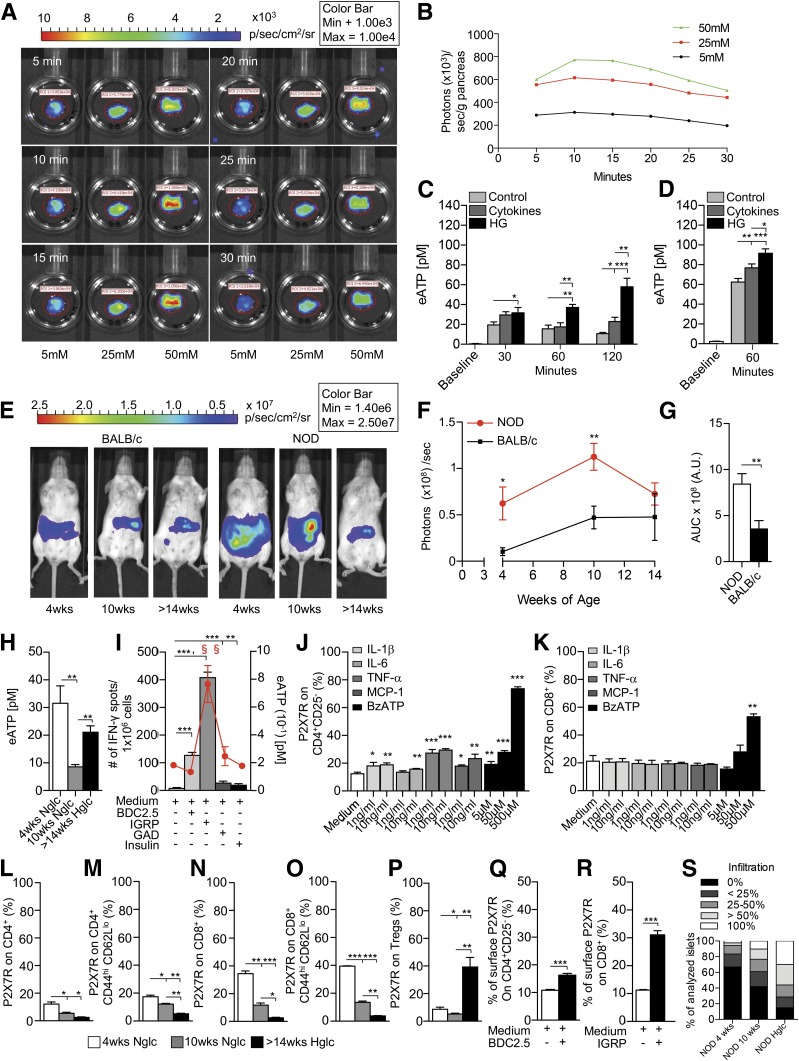
Figure 2.
β-Cells release eATP during stress. A and B: A glucose-dependent increase in the extent of luminescence (A) was evident in pancreata harvested from pmeLUC transgenic mice, cultured in the presence of different glucose concentrations and quantified as photons/seconds/grams of pancreas (B). C: Higher levels of eATP were found in the supernatants of murine islets obtained from 10-week-old NOD/SCID mice and cultured in high glucose (HG) at different time points as compared with controls. The same effect was evident to a lesser extent with a cocktail of cytokines (IL-1β, TNF-α, and IFN-γ), with high glucose being the more robust inducer of eATP. D: High-glucose conditions and cytokines (IL-1β, TNF-α, and IFN-γ) caused release of eATP by human islets cultured for 60 min. E–G: A stable luminescence signal, as a proxy for in vivo eATP release, was observed in BALB/c mice injected i.p. with HEK293-pmeLUC transgenic cells and d-luciferin at different ages, while in NOD mice the luminescence signal appeared higher than in BALB/c mice. A gradual disappearance of bioluminescence was evident over time in NOD mice, paralleling the autoimmune-mediated destruction of pancreatic islets. A.U., arbitrary units; AUC, area under the curve. H: Higher levels of eATP in peripheral serum were detected in 4-week-old NOD mice, and in hyperglycemic (Hglc) NOD mice to a lesser extent, as compared with 10-week-old NOD mice. Nglc, normoglycemic. I: During islet peptide (BDC2.5, IGRP, GAD65, and insulin) stimulation of splenocytes from NOD mice, IGRP (a CD8-restricted islet peptide) caused the highest (represented by §) release of eATP in the supernatant represented by the red line that reflects eATP release during islet peptide (BDC2.5, IGRP, GAD65, and insulin) stimulation of splenocytes from NOD mice; and the highest increase in the number of IFN-γ+ cells (represented by §) as compared with controls. BDC2.5, GAD65, and insulin peptide stimulation induced an increase in the number of IFN-γ+ spots as compared with controls as well, but to a lesser extent. J and K: CD4+CD25– T cells cultured in the presence of different concentrations of IL-1β, IL-6, TNF-α, MCP-1, or BzATP (benzoyl-ATP) showed a slight but significant upregulation of P2X7R expression on CD4+CD25– T cells, while in CD8+ T cells, BzATP only was capable of upregulating P2X7R expression. L–P: In 4-week-old NOD mice, the percentages of P2X7R+ on CD4+/CD4+ effector T cells and CD8+/CD8+ effector T cells were increased as compared with 10-week-old normoglycemic or >14-week-old hyperglycemic NOD mice. P2X7R+CD4+ effector, P2X7R+CD8+, and CD8+ effector T cells were increased in 10-week-old mice as compared with hyperglycemic NOD mice. The percentage of P2X7R+ Tregs was increased in hyperglycemic as compared with 4-week-old and 10-week-old NOD mice and in 4-week-old mive as compared with 10-week-old NOD mice (Q and R). P2X7R expression was increased in CD8+ T cells and in CD4+CD25– T cells, to a lesser extent, after culture with BDC2.5 and IGRP peptides, respectively. S: Insulitis score in 4-week-old, 10-week-old, and hyperglycemic NOD mice is shown; n = 9 sections per group were analyzed. Data are expressed as mean ± SEM. Data are representative of at least n = 3 mice. *P < 0.05; **P < 0.01; ***P < 0.001.