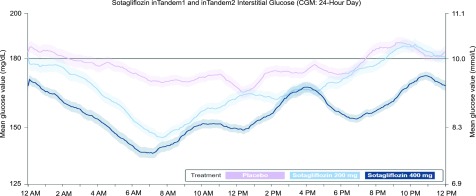
Figure 2.
Average day represented by a 24-h CGM tracing consisting of interstitial glucose readings collected every 5 min from the week prior to the week 24 visit. The figure shows data collected from midnight (0000 h). The actual start time for 24-h readings may vary for each patient. Solid lines represent mean values from each treatment group (light purple = placebo [n = 93]; light blue = sotagliflozin 200 mg [n = 89]; dark blue = sotagliflozin 400 mg [n = 96]); shaded areas represent ± 1 SEM. Top of the target CGM range = 10.0 mmol/L (180 mg/dL). The decline in mean glucose value from 12:00 to 6:00 a.m. in patients treated with sotagliflozin was not associated with a significant increase in nocturnal hypoglycemia. Ambulatory glucose profiles (AGPs) for CGM data collected from each treatment group in the week prior to week 24 appear in the Supplementary Data. Patient and aggregate visualizations of CGM data, as well as AGPs, were generated by Cenduit, LLC (Durham, NC) and any reproductions must acknowledge Cenduit.